Findings and procedure details
1. DIAGNOSTIC ALGORITHM: Accurate localization is required using two radiological principles:
-
- The Center Method: The geometric center of the lesion typically corresponds to the compartment of origin.
- The Displacement Principle: Expansile lesions displace adjacent structures away from their site of origin. Identifying which organs are displaced helps determine where the lesion did not arise.
Fig 2: Axial CT (Left) and corresponding schematic (Right) demonstrate a cystic lesion centered in the right hilum. Applying the Center Method, the geometric epicenter localizes strictly to the Visceral Compartment. (Schematic illustration created with AI assistance (Gemini/Banana Pro) and manually refined)
2. DIAGNOSTIC FRAMEWORK: A SYSTEMATIC APPROACH
We propose a four-step evaluation method applied before pattern recognition:
- CLINICAL CONTEXT
- Age
- Symptoms: Incidental vs. Compressive vs. Functional syndromes.
- MORPHOLOGICAL CHARACTERIZATION
- Laterality: Unifocal vs. Bilateral/Multifocal.
- Internal Matrix: Fluid, Fat, Calcium, or Soft Tissue.
- ENHANCEMENT PATTERN
- Vascularity: Hypervascular vs. Hypovascular vs. Avascular .
- ANATOMICAL ANCHOR
- Origin: Continuity with the spine, esophagus, or vessels.
These characteristics are summarized at the end of each case caption.
Applying this framework, we categorize the spectrum of lesions into five specific imaging patterns, detailed below with representative cases.
3. IMAGING FINDINGS: DECODING BY PATTERN
A. CYSTIC & FLUID-CONTAINING ENTITIES
-
- BRONCHOGENIC CYST:
- Pathology: Congenital malformation arising from abnormal ventral budding of the tracheobronchial tree. Lined by respiratory epithelium; contents vary from clear fluid to thick proteinaceous mucus.
- Imaging: Typically subcarinal (~50%) or paratracheal (~20%).
- CT: Variable attenuation (water to hyperdense >20HU) due to protein/calcium. Mimicking a solid mass "Pseudotumor" pitfall.
- MRI: Performed for confirmation, especially with atypical cases. T2-hyperintense (fluid) regardless of T1 signal. May show restricted diffusion if viscous [3-4].
Fig 3: Case 1 – Bronchogenic Cyst:
Panels A–D (MRI) show a cystic lesion located in the subcarinal region (arrows). The T2-weighted image without fat saturation (A) demonstrates typical fluid signal, while the T1-weighted image with fat saturation (B) reveals high signal intensity, indicative of thick or hyperproteinaceous content. Post-contrast (C) and subtraction (D) images confirm the absence of internal enhancement. Panel E (Coronal CT) shows a hypoattenuating lesion in the corresponding subcarinal region.
SYSTEMATIC APPROACH: 1. Age: Variable (Pediatric to Adulthood) | 2. Morphology: Unifocal, well-circumscribed fluid density (0–20 HU) or high attenuation (protein/calcium) | 3. Enhancement: Absent (Avascular) | 4. Anatomical Anchor: Subcarinal or paratracheal (no tracheal communication).
-
- THORACIC DUCT LYMPHOCELE:
- Pathology: Focal collection of chyle resulting from thoracic duct injury (traumatic/iatrogenic) or obstruction (mass effect).
- Imaging: Elongated, tubular or lobulated collection following the anatomic course of the thoracic duct (between aorta and azygos vein). It typically appears as a non-enhancing, multi-cystic water-density structure extending cranially from the level of the renal hila (cisterna chyli) [5-6].
- ESOPHAGEAL DUPLICATION CYST:
- Pathology: Arises from failed intrauterine vacuolization of the primitive esophagus (weeks 4–8).
- Imaging: Well-defined structure with internal fluid density running along the esophagus. Key Distinction: Its wall is typically thicker than that of a bronchogenic cyst (due to the muscular layer) [7-8].
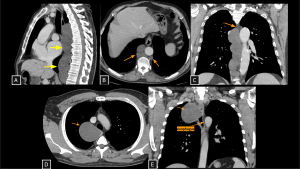
Fig 4: Cases 2 and 3 – Thoracic duct lymphocele and Esophageal Duplication Cyst:
An asymptomatic patient undergoing staging for colon neoplasia demonstrated, on chest computed tomography, an extensive elongated hypoattenuating, non-enhancing formation within the visceral mediastinum (A), communicating with small dilatations of the lumbar lymphatic ducts (arrows in B), consistent with a thoracic duct lymphocele. The lesion was secondary to an obstructive lipoma located in an anterior paravertebral position at the level of the aortic arch (arrow in C).
Panels D and E demonstrate a well-circumscribed cystic lesion in the visceral mediastinum (arrows) with broad contact with the right esophageal wall. The lesion remained stable compared to prior exams. Surgery confirmed an Esophageal Duplication Cyst.
Thoracic Duct Lymphocele SYSTEMATIC APPROACH: 1. Age: Adults (secondary to obstruction/trauma) | 2. Morphology: Elongated, multilocular water-density collection | 3. Enhancement: Absent | 4. Anatomical Anchor: Between aorta and azygos vein (thoracic duct course).
Esophageal Duplication Cyst SYSTEMATIC APPROACH: 1. Age: Variable (Pediatric to Adulthood). | 2. Morphology: Unifocal, tubular or spherical fluid-filled mass. | 3. Enhancement: Absent (Wall enhancement only). | 4. Relevant Anatomical Relationships: Intimate contact with esophageal wall (shares muscle layer). Most commonly on the right side of the distal third of the thoracic esophagus.
-
- MEDIASTINAL MULLER’S CYST (HATTORI’S CYST):
- Pathology: Rare cyst of paramesonephric duct origin, distinct from pericardial/bronchogenic cysts. Immunohistochemistry is essential: positive for Estrogen/Progesterone receptors, PAX-8 and WT1.
- Imaging: Paravertebral-visceral interface. Thin-walled, water-density, non-enhancing. Often misdiagnosed as neurogenic tumors due to the posterior location, but specifically demonstrates no communication with the neural foramen or spinal canal [9-10].
Fig 5: Case 4 – Mediastinal Muller’s Cyst:
Chest radiograph (A) demonstrates a left paravertebral opacity. Coronal (B) and axial (C) CT images show a well-defined cystic extrapulmonary lesion centered in the left posterior mediastinum, in contact with the aorta and adjacent vertebral bodies at the level of the 5th and 6th costal arches, without evidence of invasion. Histopathological analysis with immunohistochemistry (D, E, and F) demonstrates positivity for estrogen receptor, progesterone receptor, and PAX-8, respectively, confirming the diagnosis of a mediastinal Müllerian cyst.
SYSTEMATIC APPROACH: 1. Age: Adult Females. | 2. Morphology: Thin-walled, water-density, non-enhancing cyst. | 3. Enhancement: Absent. | 4. Relevant Anatomical Relationships: Paravertebral-visceral interface; specifically demonstrates no communication with the neural foramen or spinal canal.
-
- LATERAL MENINGOCELE:
- Pathology: Protrusion of arachnoid/dura mater through an enlarged intervertebral foramen. Strong association with Neurofibromatosis Type 1 (NF1) due to dural dysplasia.
- Imaging: Well-circumscribed paravertebral lesion with CSF attenuation. Smooth enlargement of the neural foramen may be present. Occasionally shows thin peripheral enhancement, mimicking cystic neoplasms [11-12].
Fig 6: Case 5 - Lateral Thoracic Meningocele:
Panel A (coronal CT image) demonstrates a large, well-defined, low-attenuation paravertebral cystic lesion extending into the left hemithorax, causing mass effect on adjacent structures. Panel B (axial CT image) shows a focal communication between the lesion and the spinal canal through an enlarged neural foramen (yellow arrow), a key imaging feature confirming the diagnosis of lateral thoracic meningocele.
SYSTEMATIC APPROACH: 1. Age: Variable (Assoc. with NF1). | 2. Morphology: Well-circumscribed paravertebral lesion with CSF attenuation. | 3. Enhancement: None (occasionally thin peripheral). | 4. Relevant Anatomical Relationships: Protrusion through an enlarged intervertebral foramen.
B. NEUROGENIC & SPINDLE CELL TUMORS
-
- PERIPHERAL NERVE SHEATH TUMORS (PNST):
- Pathology: Arise from Schwann cells (Schwannoma) or a mixture of Schwann cells and fibroblasts (Neurofibroma). Schwannomas grow eccentrically to the nerve, while Neurofibromas cause fusiform nerve expansion. Malignant peripheral nerve sheath tumor (MPNST) represents malignant transformation, strongly associated with NF1.
- Imaging: Ovoid, spherical, or dumbbell-shaped masses with foraminal extension. May arise from visceral nerves (vagus or phrenic), presenting as visceral mediastinal masses. Imaging features suggesting malignancy include heterogeneity, necrosis and invasive behavior [13-14].
- GANGLIONEUROMA:
- Pathology: Benign, mature tumor arising from sympathetic ganglion cells (distinct from nerve sheath tumors) [15].
- Imaging: Elongated, vertically oriented paravertebral mass spanning multiple vertebral levels. Characteristic “scimitar” configuration. Tends to partially encase vessels without luminal narrowing, unlike bulky PNSTs.
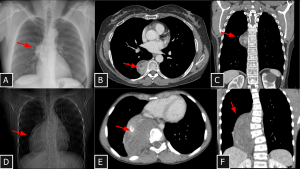
Fig 7: Cases 6 and 7 – Neurogenic tumors of the mediastinum (Case 6: A, B, and C and Case 7: D, E, and F): Chest radiographs show right paravertebral masses (A and D). Both lesions demonstrate heterogeneous attenuation (see C and F), one with hypoattenuating foci—likely cystic components (arrow in B)—and the other with calcified foci (arrow in E). Bone remodeling is also observed as an associated finding. Biopsies confirmed schwannoma and ganglioneuroma, respectively.
Schwannoma SYSTEMATIC APPROACH: 1. Age: Adults (20–50 years). | 2. Morphology: Solitary, encapsulated, spherical with cystic degeneration. | 3. Enhancement: Heterogeneous. | 4. Relevant Anatomical Relationships: Paravertebral / intercostal space, following the nerve anatomical orientation; eccentric to the nerve root.
Ganglioneuroma SYSTEMATIC APPROACH: 1. Age: Children and Young Adults. | 2. Morphology: Vertically oriented, oblong/crescentic mass. | 3. Enhancement: Gradual/Delayed (progressive). | 4. Relevant Anatomical Relationships: Paravertebral; tends to surround vessels without narrowing them.
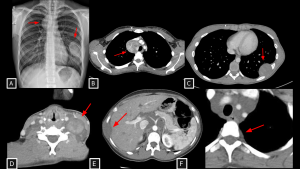
Fig 8: Cases 8 and 9 – Malignant neural-lineage neoplasm presenting as a visceral mediastinal mass and Neurofibromas.
A 21-year-old patient with a history of neurofibromatosis type 1 undergoing evaluation for cough and constitutional symptoms. Chest radiograph (A) showed mediastinal widening and a left pulmonary mass. Corresponding to the radiographic findings, contrast-enhanced chest CT (B and C) demonstrated two heterogeneous expansile lesions located in the visceral mediastinum and left thoracic wall, containing hypoattenuating foci (likely cystic components). Biopsy of the right paratracheal lesion revealed a malignant peripheral nerve sheath tumor.
Case 9 (D–F) demonstrates disseminated plexiform neurofibromas: a neck mass splaying carotid space vessels without stenosis (D), multiple fusiform hypoattenuating intercostal lesions (E), and extensive retroperitoneal soft tissue encasing the aorta and celiac trunk (F).
MPNST (Malignant Peripheral Nerve Sheath Tumor) SYSTEMATIC APPROACH: 1. Age: Adults (increased risk in NF1). | 2. Morphology: Large, ill-defined mass with necrosis/hemorrhage. | 3. Enhancement: Heterogeneous/Peripheral. | 4. Relevant Anatomical Relationships: Invasive; rapid growth with destruction of adjacent planes.
Neurofibroma SYSTEMATIC APPROACH: 1. Age: Pediatric/Young Adults (NF1 context). | 2. Morphology: Diffuse, infiltrative ("Bag of worms"). | 3. Enhancement: Variable. | 4. Relevant Anatomical Relationships: Multicompartmental; involves nerve plexuses/branches.
-
- ESOPHAGEAL LEIOMYOMA:
- Pathology: Benign mesenchymal tumor arising from smooth muscle cells of the esophageal muscularis propria. Represents the most common benign tumor of the esophagus.
- Imaging: Homogeneous soft-tissue attenuation. Intramural, eccentric mass forming obtuse angles with the esophageal wall. Lack of mucosal ulceration and lymphadenopathy differentiates from carcinoma. Coarse calcifications are rare but highly specific, particularly at the gastroesophageal junction [16].
Fig 9: Cases 10 - Esophageal leiomyoma.
Panels A and B depict a homogeneous solid nodule located anterior to the esophageal wall (visceral mediastinum) (arrows in A and B), consistent with an esophageal leiomyoma previously identified on upper endoscopy.
SYSTEMATIC APPROACH: 1. Age: Adults. | 2. Morphology: Homogeneous soft-tissue attenuation; intramural, eccentric mass. | 3. Enhancement: Homogeneous / Mild. | 4. Relevant Anatomical Relationships: Forms obtuse angles with the esophageal wall; lack of mucosal ulceration.
C. HYPERVASCULAR & LYMPHOPROLIFERATIVE
-
- PARAGANGLIOMA:
- Pathology: Neuroendocrine tumor arising from extra-adrenal chromaffin cells (sympathetic chain). ~40% secrete catecholamines (functional). Highly vascular network.
- Imaging: Intense, vivid arterial enhancement on CT with rapid washout. Avid uptake on MIBG or 68-Ga-DOTATATE-PET. Enhancement is significantly higher than in Schwannomas [17-19].
Fig 10: Case 11 – Paravertebral Paraganglioma:
Unenhanced CT (A) reveals a paravertebral mass extending from the retroperitoneum into the thorax. The intense arterial enhancement seen in Panel B is the key diagnostic feature, confirming the tumor's hypervascular nature and distinguishing it from other neurogenic lesions.
SYSTEMATIC APPROACH: 1. Age: Adults (40–50 years; younger in hereditary cases). | 2. Morphology: Highly vascular soft-tissue mass ("Salt-and-pepper" on MRI). | 3. Enhancement: Intense/Avid arterial enhancement. | 4. Relevant Anatomical Relationships: Paravertebral (Sympathetic chain); intense uptake on DOTATATE/MIBG.
-
- CASTLEMAN DISEASE (UNICENTRIC):
- Pathology: A heterogeneous group of lymphoproliferative disorders that share common morphological features on lymph node biopsy. Hyaline-Vascular type (90%) characterized by follicular hyperplasia and marked interfollicular vascularity.
- Imaging: Solitary, non-necrotic mass. Intense homogeneous enhancement on contrast-enhanced CT (mimicking paraganglioma) [20].
Fig 11: Case 12 – Castleman Disease:
Axial CT (A) reveals a right hilar mass with extension to the ipsilateral posterior mediastinum. Coronal view (B) demonstrates the anatomical relationship with the bronchi. The lesion showed uptake on 18 FDG PET CT (C) but remained stable over a 3-year period, consistent with this benign lymphoproliferative disorder.
SYSTEMATIC APPROACH: 1. Age: Young Adults / Adults. | 2. Morphology: Solitary, non-necrotic mass. | 3. Enhancement: Intense homogeneous enhancement (mimicking paraganglioma). | 4. Relevant Anatomical Relationships: Unicentric distribution; often visceral or vascular interface.
-
- ECTOPIC PARATHYROID ADENOMA:
- Pathology: Abnormal migration of parathyroid tissue, commonly involving inferior glands.
- Imaging: Oval, enhancing nodule in the visceral compartment. Confirm with 99mTc-Sestamibi SPECT/CT or 4D-CT (wash-in/wash-out kinetics) [21].
Fig 12: Case 13 – Ectopic parathyroid gland:
Panels A, B, and C demonstrate an elongated, well-defined solid nodule posterior to the esophageal wall (visceral mediastinum) (arrows in A and B), with apparent arterial supply originating near the thyroid gland. This lesion was identified as a hyperfunctioning ectopic parathyroid gland on technetium-99m sestamibi scintigraphy (C).
SYSTEMATIC APPROACH: 1. Age: Adults. | 2. Morphology: Oval nodule. | 3. Enhancement: Avid enhancement (Wash-in/Wash-out). | 4. Relevant Anatomical Relationships: Visceral compartment (along the migration pathway of the glands).
-
- PRIMARY CARDIAC LYMPHOMA:
- Pathology: Extremely rare extranodal Non-Hodgkin Lymphoma (B-cell type). Typically affects immunocompromised patients. Involves the right heart (Right Atrium) and pericardium.
- Imaging: Infiltrating mass involving multiple chambers and pericardium. Tendency to encase structures rather than simply displacing them [22].
Fig 13: Case 14 – Cardiac Lymphoma:
Axial (A) and coronal (B) CT Coronary Angiography reveal hypoattenuating soft-tissue lesions encasing the cardiac chambers. Volume Rendering reconstructions (C and D) provide a 3D perspective of the tumor burden, clearly depicting the relationship between the masses and the heart chambers.
SYSTEMATIC APPROACH: 1. Age: Immunocompromised or Elderly. | 2. Morphology: Infiltrative, homogeneous soft-tissue mass. | 3. Enhancement: Mild to moderate (Homogeneous). | 4. Relevant Anatomical Relationships: Right Atrium and pericardium; encases coronary vessels.
D. MATRIX CHARACTERIZATION (FAT, BLOOD, BONE)
-
- EXTRAMEDULLARY HEMATOPOIESIS:
- Pathology: Compensatory production of blood cells outside marrow in chronic anemia (Thalassemia, Sickle Cell).
- Imaging: Bilateral, lobulated paravertebral masses (lower thoracic). Soft tissue mixed with macroscopic fat (chronic). Causes smooth cortical remodeling/expansion without aggressive erosion/destruction [23].
Fig 14: Case 15 – Extramedullary hematopoiesis related to sickle cell anemia:
Panels A, B, and C: Widening of the posterior mediastinum behind the heart is seen on the chest radiograph (A), demonstrated on chest CT as bilateral, nearly symmetrical paravertebral masses with soft-tissue attenuation and scattered foci of fat attenuation (arrows in B). The bone window on chest CT (C) revealed heterogeneous trabecular bone texture and H-shaped vertebral bodies, findings suggestive of sickle cell anemia, which correlates with the extramedullary hematopoiesis presenting as paravertebral masses in this case.
SYSTEMATIC APPROACH: 1. Age: Adults. | 2. Morphology: Bilateral, lobulated masses; soft tissue mixed with macroscopic fat. | 3. Enhancement: Mild / Heterogeneous. | 4. Relevant Anatomical Relationships: Paravertebral (lower thoracic); causes smooth cortical remodeling/expansion without aggressive erosion.
-
- LIPOSARCOMA:
- Pathology: Malignant tumor of adipocytic differentiation.
- Imaging: Heterogeneous mass with areas of macroscopic fat and solid enhancing components. Direct invasion of adjacent structures is common [24].
Fig 15: Case 16 - Liposarcoma:
Panel A (coronal CT image) demonstrates a large heterogeneous solid mass occupying the middle and lower portions of the left hemithorax, with features suggestive of posterior mediastinal origin. Panel B (axial CT image) shows the lesion containing mixed components of macroscopic fat and soft-tissue attenuation, causing rightward displacement of the esophagus and close contact with the descending thoracic aorta and left atrium, imaging features consistent with mediastinal liposarcoma.
SYSTEMATIC APPROACH: 1. Age: Adults. | 2. Morphology: Heterogeneous mass with areas of macroscopic fat and solid components. | 3. Enhancement: Heterogeneous (solid areas). | 4. Relevant Anatomical Relationships: Invasive behavior; direct invasion of adjacent structures is common.
-
- PARAVERTEBRAL HEMATOMA:
- Pathology: Accumulation of blood due to trauma, coagulopathy or vascular rupture.
- Imaging: High attenuation on CT (50–70 HU) in acute phase (separates it from soft tissue tumors). Displaces mediastinal pleura laterally (extra-pleural sign). May show active contrast extravasation [25].
Fig 16: Case 17 – Paravertebral hematoma in trauma:
A 27-year-old patient who sustained a 10-meter fall presented with multiple vertebral body fractures from T8 to T11 (arrows in C) and a large paravertebral mediastinal hematoma extending from T5 to T11 (B), causing anterior displacement of visceral mediastinal structures. An arterial focus of active bleeding was identified (arrows in A).
SYSTEMATIC APPROACH: 1. Age: Any (Trauma/Coagulopathy). | 2. Morphology: High attenuation on CT (50–70 HU) in acute phase. | 3. Enhancement: None (except active extravasation). | 4. Relevant Anatomical Relationships: Paravertebral; displaces mediastinal pleura laterally (extra-pleural sign).
-
- TUBERCULOUS SPONDYLODISCITIS (POTT’S DISEASE):
- Pathology: Hematogenous spread of M. tuberculosis to spine. Extension into soft tissues (cold abscess).
- Imaging: Destruction of vertebral endplates and loss of disc height (unlike metastatic preservation of disc) [26,27].
Fig 17: Case 18 – Tuberculous spondylodiscitis:
Panels A, B, and C: Sequelae of tuberculosis are indicated by fibroatelectatic streaks and irregular pulmonary nodules in the lung apices (A). Lytic lesions are seen in the vertebral bodies (T6–T12) (B). In addition, there are heterogeneous paravertebral collections adjacent to the affected vertebral bodies (C), representing an important differential diagnosis for paravertebral mediastinal masses.
SYSTEMATIC APPROACH: 1. Age: Any. | 2. Morphology: Vertebral destruction with soft tissue component (cold abscess). | 3. Enhancement: Peripheral (rim enhancement). | 4. Relevant Anatomical Relationships: Destruction of vertebral endplates and disc involvement (unlike metastatic preservation of disc).
-
- GIANT FIBROVASCULAR POLYP OF THE ESOPHAGUS:
- Pathology: Rare benign intraluminal lesion (<2%) arising from the cervical esophagus (cricopharyngeus level). Composed of fibrous tissue, blood vessels, and adipose tissue.
- Imaging: Elongated, "sausage-like", well-defined mass expanding the esophageal lumen attached by a peduncle. Definitive diagnosis requires MDM2 amplification testing to exclude well-differentiated liposarcoma [28,29].
Fig 18: Case 19 – Giant Fibrovascular Polyp of the Esophagus:
Panels A and B (Sagittal and Coronal CT, respectively) demonstrate a large intraluminal lesion originating in the cervical esophagus, at the level of the cricopharyngeus muscle. The mass extends distally and presents heterogeneous attenuation.
SYSTEMATIC APPROACH: 1. Age: Elderly males. | 2. Morphology: Sausage-like intraluminal mass with macroscopic fat. | 3. Enhancement: Heterogeneous (vessels/fibrous septa). | 4. Relevant Anatomical Relationships: Originates in cervical esophagus (Cricopharyngeus); extends distally.
E. SYSTEMIC, SECONDARY & RARE CONDITIONS
-
- IgG4-RELATED DISEASE:
- Pathology: Systemic fibro-inflammatory condition characterized by lymphoplasmacytic infiltration rich in IgG4+ plasma cells, obliterative phlebitis and storiform fibrosis.
- Imaging: Diffuse soft-tissue thickening or mass-like "fibrosing mediastinitis". Hallmark: Encasement of paravertebral/mediastinal vessels without luminal occlusion or frank invasion [30,31].
Fig 19: Case 20 – Paravertebral Lesions in IgG4-Related Disease:
Paravertebral lesions with soft-tissue attenuation were identified on CT and radiography, extending anteriorly and to the right of the thoracic vertebral bodies (B and C), without sclerotic or lytic bone changes. The lesions encased the intercostal vessels (A) without causing compression or infiltration.
The imaging findings were suggestive of IgG4-related paravertebral disease, later confirmed by biopsy.
SYSTEMATIC APPROACH: 1. Age: Middle-aged to Elderly males. | 2. Morphology: Diffuse soft-tissue thickening ("Rind-like"). | 3. Enhancement: Homogeneous delayed enhancement. | 4. Relevant Anatomical Relationships: Paravertebral/Periaortic; encases vessels without luminal stenosis.
-
- MEDIASTINAL METASTASIS:
- Pathology: Predominantly lymphatic spread or hematogenous.
- Imaging: Conglomerate lymphadenopathy (often necrotic/bulky). Osteolytic destruction indicates aggressive invasion. Differentiation relies on multiplicity and known primary history [32].
Fig 20: Case 21 – Paravertebral mediastinal metastasis:
A 35-year-old patient with an atypical lipomatous tumor of the right leg (previously operated, with local recurrence demonstrated on MRI (A) developed a heterogeneous solid lesion in the paravertebral mediastinum (arrow in B). The lesion was located anterior and left to the thoracic vertebral bodies and contained foci of fat attenuation (arrows in B and C), consistent with secondary involvement.
SYSTEMATIC APPROACH: 1. Age: Adults / Elderly (History of primary malignancy). | 2. Morphology: Osteolytic bone destruction or conglomerate nodal mass. | 3. Enhancement: Variable (depends on primary). | 4. Relevant Anatomical Relationships: Destroys vertebral body or invades adjacent organs.
-
- MEGAESOPHAGUS:
- Pathology: Functional motor disorder (Chagas, Achalasia) leading to massive dilation.
- Imaging: On radiography, appears as a widened mediastinum with air-fluid level. Mimics a solid mass on non-contrast or scout images; oral contrast confirms diagnosis [33].
Fig 21: Case 22 – Megaesophagus:
Posteroanterior (A) and lateral (B) chest radiographs of a female patient with Chagas disease show mediastinal widening, anterior displacement of the trachea, and an elongated retrocardiac opacity. A barium oral contrast esophagogram (C) demonstrated a dolichomegaesophagus mimicking a mediastinal mass.
SYSTEMATIC APPROACH: 1. Age: Adults (e.g., Chagas, Achalasia). | 2. Morphology: Widened mediastinum with air-fluid level. | 3. Enhancement: N/A (Viscus). | 4. Relevant Anatomical Relationships: Visceral compartment; mimics a solid mass on non-contrast images.
-
- CARDIAC MYXOMA:
- Pathology: Most common primary cardiac tumor. Benign, occurring in adults (30-60y, F>M). Predilection for the Left Atrium (75%) attached to the fossa ovalis of the interatrial septum.
- Imaging: Hypodense intracavitary mass. Myxomas arise from the fossa ovalis and demonstrate gadolinium enhancement. In contrast, thrombi are typically situated in the posterior appendage and do not enhance [22,34].
Fig 22: Case 23 – Cardiac Myxoma:
Cardiac MRI (A, B, C, E, F) reveals a lobulated mass in the left atrium. Note the high signal intensity on bright-blood sequences (circle in A) due to the mucinous/water-rich content, which can mimic a cyst. However, the lesion presents heterogeneous enhancement (circle in F), confirming it is a solid neoplasm and ruling out avascular thrombus. Echocardiogram (D) demonstrates the classic pedunculated attachment to the interatrial septum/fossa ovalis (arrow).
SYSTEMATIC APPROACH: 1. Age: Adults (30–60 years). | 2. Morphology: Lobulated, low-attenuation mass (mimics fluid). | 3. Enhancement: Heterogeneous (Solid). | 4. Relevant Anatomical Relationships: Left Atrium; attached to the Fossa Ovalis (Interatrial septum).
-
- MEDIASTINAL AMYLOIDOMA:
- Pathology: Localized form of amyloidosis characterized by the extracellular deposition of insoluble fibrillar proteins.
- Imaging: Can present as specific soft-tissue masses or diffuse mediastinal infiltration.Often exhibits calcifications (punctate, diffuse or "bizarre-shaped") and typically shows significant stability over long periods [35].
Fig 23: Case 24 – Mediastinal Amyloidoma:
Coronal, sagittal and axial CT images demonstrate extensive confluent lymphadenopathy with coarse calcifications involving the right cervical, superior mediastinal, and subcarinal chains (A, B and C). The lesion displaces the mediastinal structures to the left. Chest radiography (D) correlates with these findings, showing mediastinal widening.
SYSTEMATIC APPROACH: 1. Age: Adults/Elderly. | 2. Morphology: Soft-tissue mass with calcifications (punctate/diffuse). | 3. Enhancement: Variable/Delayed. | 4. Relevant Anatomical Relationships: Diffuse infiltration or focal mediastinal/hilar masses.






















