Müllerian Duct Anomalies (MDA)
MDAs are congenital abnormalities that arise from the improper development, fusion, or resorption of the embryological structures responsible for forming the female reproductive tract. These anomalies can affect the uterus, cervix, and upper vagina, and their impact on fertility and pregnancy outcomes varies depending on the specific type (1, 2, 4, 6).
MRI has emerged as the gold standard in detecting and classifying MDAs due to its ability to provide detailed images of uterine anatomy, particularly the external fundal contour (3).
Types of MDAs and their MRI findings
-
Müllerian agenesis: the uterus, cervix, and upper vagina are completely absent. MRI shows bilateral rudimentary uteri and can detect associated renal anomalies, such as ectopic kidneys or renal agenesis. One of the most recognized forms of this anomaly is Mayer-Rokitansky-Küster-Hauser Syndrome (MRKHS), often presenting with primary amenorrhea in young women (Figure 1).
 Fig 1: (A–C) MRKHS with rudimentary uterine horns and vaginal agenesis in an 18-year-old woman. Axial T2-weighted MR image (A) shows fat in the expected location of the lower vagina (arrow). Coronal T2-weighted MR image (B) shows bilateral rudimentary uterine horns (arrows). Axial T2-weighted MR image (C) shows low-signal-intensity fibrous bands (arrows), extending from the rudimentary horns to the midline, which is a common feature in these patients. Coronal T2weighted MR image (D) in a 30-year-old woman with MRKHS shows bilateral pelvic kidneys (arrowheads) and abnormal positioning of the left ovary in the left paracolic gutter (arrow).
Fig 1: (A–C) MRKHS with rudimentary uterine horns and vaginal agenesis in an 18-year-old woman. Axial T2-weighted MR image (A) shows fat in the expected location of the lower vagina (arrow). Coronal T2-weighted MR image (B) shows bilateral rudimentary uterine horns (arrows). Axial T2-weighted MR image (C) shows low-signal-intensity fibrous bands (arrows), extending from the rudimentary horns to the midline, which is a common feature in these patients. Coronal T2weighted MR image (D) in a 30-year-old woman with MRKHS shows bilateral pelvic kidneys (arrowheads) and abnormal positioning of the left ovary in the left paracolic gutter (arrow). -
Unicornuate uterus: one Müllerian duct fails to develop, resulting in a single elongated uterine cavity. This may include a rudimentary horn, which can cause cyclic pain or obstruction (Figure 2).
 Fig 2: Developmental MDAs. (A) 3D US image (with corresponding illustration, top) of a unicornuate uterus in a 32-year-old woman that was acquired during saline solution infused sonohysterography shows a single fluid-filled uterine cavity (arrow). (B) Axial T2weighted MR image (with corresponding illustration, top) in a 36-year-old woman shows a unicornuate left uterus (solid arrow) and an atretic noncommunicating right uterine horn (dashed arrow).
Fig 2: Developmental MDAs. (A) 3D US image (with corresponding illustration, top) of a unicornuate uterus in a 32-year-old woman that was acquired during saline solution infused sonohysterography shows a single fluid-filled uterine cavity (arrow). (B) Axial T2weighted MR image (with corresponding illustration, top) in a 36-year-old woman shows a unicornuate left uterus (solid arrow) and an atretic noncommunicating right uterine horn (dashed arrow). -
Uterus Didelphys: complete failure of fusion results in two separate uteri, each with its endometrium, myometrium, and often a double cervix. Fertility is generally preserved, but there are higher risks of miscarriage and preterm birth (Figure3).
 Fig 3: MR images (with corresponding illustrations, top) of MDAs of absent or incomplete fusion. (A) Coronal T2-weighted MR image of a uterus didelphys in a 26-year-old woman shows two separate unicornuate uterine cavities (arrows) and a double cervix (arrowheads). (B) Coronal T2-weighted MR image of a bicornuate uterus in a 31-year-old woman shows external indentation of the uterine fundal contour that is slightly larger than 1 cm, with a septum extending to the level of the internal cervical os (arrowhead).
Fig 3: MR images (with corresponding illustrations, top) of MDAs of absent or incomplete fusion. (A) Coronal T2-weighted MR image of a uterus didelphys in a 26-year-old woman shows two separate unicornuate uterine cavities (arrows) and a double cervix (arrowheads). (B) Coronal T2-weighted MR image of a bicornuate uterus in a 31-year-old woman shows external indentation of the uterine fundal contour that is slightly larger than 1 cm, with a septum extending to the level of the internal cervical os (arrowhead). -
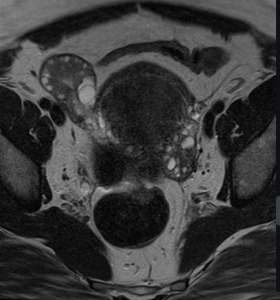
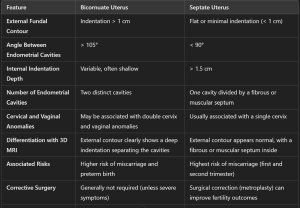
Bicornuate uterus: partial fusion failure leads to two endometrial cavities separated by a shared myometrium. MRI shows a wide-angle divergence (>105°)between the cavities and a prominent external indentation (>1 cm) (Figure 4).
 Fig 4: MR images (with corresponding illustrations, top) show classification of normal or arcuate (A), septate (B), and bicornuate (C) uteri on the basis of ASRM 2016 criteria, which apply for both complete septate and partial septate uteri. (A) Axial oblique T2-weighted MR image of a normal or arcuate uterus in a 33-year-old woman shows a depth of internal indentation (solid line) of less than 1 cm (double-headed arrow) and an angle of indentation of greater than 90°. (B) Axial T2-weighted MR image of a septate uterus in a 31-year-old woman shows an internal indentation (solid line) of greater than 1.5 cm (double-headed arrow), an angle of indentation of less than 90°, and a flat external uterine contour (dashed line). (C) Axial oblique T2-weighted MR image of a bicornuate uterus in a 37-year-old transgender man shows external uterine fundal indentation (dashed line) of greater than 1 cm (dashed double-headed arrow).
Fig 4: MR images (with corresponding illustrations, top) show classification of normal or arcuate (A), septate (B), and bicornuate (C) uteri on the basis of ASRM 2016 criteria, which apply for both complete septate and partial septate uteri. (A) Axial oblique T2-weighted MR image of a normal or arcuate uterus in a 33-year-old woman shows a depth of internal indentation (solid line) of less than 1 cm (double-headed arrow) and an angle of indentation of greater than 90°. (B) Axial T2-weighted MR image of a septate uterus in a 31-year-old woman shows an internal indentation (solid line) of greater than 1.5 cm (double-headed arrow), an angle of indentation of less than 90°, and a flat external uterine contour (dashed line). (C) Axial oblique T2-weighted MR image of a bicornuate uterus in a 37-year-old transgender man shows external uterine fundal indentation (dashed line) of greater than 1 cm (dashed double-headed arrow). - Septate uterus: incomplete resorption of the central uterine septum causes a fibrous or muscular partition within the cavity. MRI reveals a deep internal indentation (>1.5 cm) of the endometrium with a normal external contour (< 1 cm) (Figure 5).
 Fig 5: MR images (with corresponding illustrations, top) of MDAs related to failure of resorption (A, B) compared with a normal uterus (C). (A) Axial oblique T2-weighted MR image of a septate uterus in a 34-year-old woman shows a convex uterine fundal contour (dashed line), internal indentation of greater than 1.5 cm (double-headed arrow) from the interostial line (solid line) to the apex of the indentation of the endometrial cavity, and a fibrous septum extending to the level of the internal cervical os (arrow). (B) Axial T2-weighted MR image of a partial septate uterus in a 36-year-old woman shows a convex uterine fundal contour (dashed line) and internal indentation of greater than 1.5 cm (double-headed arrow). (C) Axial T2-weighted MR image of a normal or arcuate uterus in a 35-year-old woman shows a convex uterine fundal contour (dashed line) without significant internal indentation (<1 cm).
Fig 5: MR images (with corresponding illustrations, top) of MDAs related to failure of resorption (A, B) compared with a normal uterus (C). (A) Axial oblique T2-weighted MR image of a septate uterus in a 34-year-old woman shows a convex uterine fundal contour (dashed line), internal indentation of greater than 1.5 cm (double-headed arrow) from the interostial line (solid line) to the apex of the indentation of the endometrial cavity, and a fibrous septum extending to the level of the internal cervical os (arrow). (B) Axial T2-weighted MR image of a partial septate uterus in a 36-year-old woman shows a convex uterine fundal contour (dashed line) and internal indentation of greater than 1.5 cm (double-headed arrow). (C) Axial T2-weighted MR image of a normal or arcuate uterus in a 35-year-old woman shows a convex uterine fundal contour (dashed line) without significant internal indentation (<1 cm).
MRI is particularly valuable in distinguishing between a bicornuate uterus and a septate uterus, which require different management strategies. In a septate uterus, the angle between cavities is <90°, whereas a bicornuate uterus shows a wider angle (5). (Table 1)
Fibroids (Leiomyomas)
Fibroids are benign myometrial tumours, affecting up to 70% of women of reproductive age. Although they are a rare cause of infertility, fibroids can interfere with implantation by distorting the uterine cavity or compressing the fallopian tubes. Submucosal fibroids have the greatest impact on fertility (17, 18).
MRI provides superior accuracy in mapping and characterising fibroids compared to TV-US. It can identify the fibroid type (subserosal, intramural, submucosal, or extrauterine) and quantify the degree of submucosal extension. This information is vital for planning surgical interventions such as hysteroscopic resection. Additionally, MRI distinguishes fibroids from adenomyosis, which requires different treatments (14).
Fibroids and their MRI findings
Conventional leiomyomas: homogeneous, low signal intensity on T2-weighted images, and isointense or mildly hypointense on T1-weighted images. Show early homogeneous enhancement post-contrast (15, 16). Based on their localization and following the FIGO classification system (Figures 6 and 7), fibroids are distinguished into:
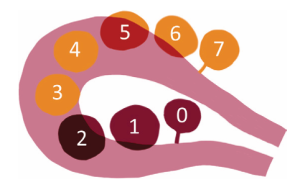
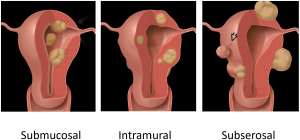
- Subserosal Leiomyomas (FIGO Stages 5-7): located on the outer surface of the uterus. Well-circumscribed masses projecting outward from the uterine serosa. Bridging vessels between the uterus and the mass are a key diagnostic feature. Pedunculated subserosal leiomyomas may show a "claw sign." (Figure 8)
 Fig 8: Subserosal Leiomyomas (FIGO Stages 5-7): located on the outer surface of the uterus. Well-circumscribed masses projecting outward from the uterine serosa. Bridging vessels between the uterus and the mass are a key diagnostic feature. Pedunculated subserosal leiomyomas may show a "claw sign."
Fig 8: Subserosal Leiomyomas (FIGO Stages 5-7): located on the outer surface of the uterus. Well-circumscribed masses projecting outward from the uterine serosa. Bridging vessels between the uterus and the mass are a key diagnostic feature. Pedunculated subserosal leiomyomas may show a "claw sign." - Intramural Leiomyomas (FIGO Stages 3-4): located within the myometrium. Low T2 signal intensity relative to the myometrium. Often homogeneous unless degeneration is present. Show early homogeneous enhancement post-contrast. (Figure 9)
 Fig 9: Intramural Leiomyomas (FIGO Stages 3-4): located within the myometrium. Low T2 signal intensity relative to the myometrium. Often homogeneous unless degeneration is present. Show early homogeneous enhancement post-contrast.
Fig 9: Intramural Leiomyomas (FIGO Stages 3-4): located within the myometrium. Low T2 signal intensity relative to the myometrium. Often homogeneous unless degeneration is present. Show early homogeneous enhancement post-contrast. - Submucosal Leiomyomas (FIGO Stages 0-2): located beneath the endometrium and protrudes into the uterine cavity. Show a T2-hypointense mass in the endometrial cavity. These tumors can appear with a T2-hyperintense rim due to edema or obstructed veins. (Figures 10 and 11)
 Fig 10: Submucosal Leiomyomas (FIGO Stages 0-2): located beneath the endometrium and protrudes into the uterine cavity. Show a T2-hypointense mass in the endometrial cavity. These tumors can appear with a T2-hyperintense rim due to edema or obstructed veins.
Fig 10: Submucosal Leiomyomas (FIGO Stages 0-2): located beneath the endometrium and protrudes into the uterine cavity. Show a T2-hypointense mass in the endometrial cavity. These tumors can appear with a T2-hyperintense rim due to edema or obstructed veins. Fig 11: Submucosal Leiomyomas (FIGO Stages 0-2). On the left: nondegenerated conventional leiomyoma in a 32-year-old woman. (A) Sagittal T2W MR image shows a round T2-hypointense leiomyoma (arrow) of the anterior uterine body (FIGO stage 1). On the right: FIGO stage 0 leiomyoma in a 42-year-old woman who presented with heavy menses. Sagittal T2W MR image shows a hypointense pedunculated conventional leiomyoma (arrow) extending from the lower uterine segment into the upper vagina. The thin stalk (arrowhead) can be seen within the endocervical canal. There is also a FIGO stage 1 submucosal leiomyoma partially imaged in the superior aspect of the endometrium.
Fig 11: Submucosal Leiomyomas (FIGO Stages 0-2). On the left: nondegenerated conventional leiomyoma in a 32-year-old woman. (A) Sagittal T2W MR image shows a round T2-hypointense leiomyoma (arrow) of the anterior uterine body (FIGO stage 1). On the right: FIGO stage 0 leiomyoma in a 42-year-old woman who presented with heavy menses. Sagittal T2W MR image shows a hypointense pedunculated conventional leiomyoma (arrow) extending from the lower uterine segment into the upper vagina. The thin stalk (arrowhead) can be seen within the endocervical canal. There is also a FIGO stage 1 submucosal leiomyoma partially imaged in the superior aspect of the endometrium. - Extrauterine Leiomyomas (FIGO Stage 8): found outside the uterus, such as in the cervix, broad ligament, or parasitic leiomyomas. Typically have imaging characteristics similar to uterine leiomyomas.
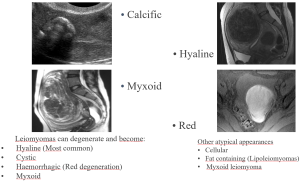
Degenerated leiomyomas: leiomyomas can undergo one or several types of degeneration that may alter their imaging appearance (Figure 12).
Adenomyosis
Adenomyosis occurs when endometrial glands infiltrate the myometrium. It can coexist with endometriosis and may cause infertility by interfering with implantation (8).
MRI findings in adenomyosis:
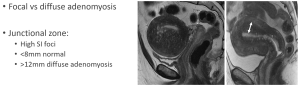
- Location: diffuse or focal thickening of the junctional zone (JZ) of the uterus.
- T2-weighted images: thickened junctional zone (>12 mm) with poorly defined borders. Hyperintense myometrial cysts are a hallmark feature.
- T1-weighted images: small hyperintense foci representing hemorrhagic foci within ectopic endometrial tissue.
- Post-contrast: shows poor or heterogeneous enhancement of the thickened JZ (Figure 13).
Key differences in MRI between fibroids and adenomyosis
- Fibroids: well-circumscribed masses with low T2 signal intensity and early homogeneous enhancement.
- Adenomyosis: diffuse thickening of the junctional zone with hyperintense myometrial cysts on T2 and poorly defined borders (11). (Figure 14), (Table 2)
 Fig 14: Key differences in MRI between fibroids and adenomyosis.
Fig 14: Key differences in MRI between fibroids and adenomyosis. Table 2: Key differences in MRI between fibroids and adenomyosis.
Table 2: Key differences in MRI between fibroids and adenomyosis.
Endometriosis
Endometriosis is characterised by the presence of endometrial tissue outside the uterus, affecting 30-50% of women with infertility. The condition can lead to chronic pelvic pain, dyspareunia, and dysmenorrhea. Endometriosis impacts fertility by causing peritubal adhesions, blocked fallopian tubes, and impaired implantation. (Figure 15)
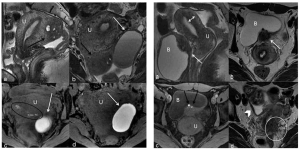
MRI in endometriosis
MRI is effective in detecting endometriosis, particularly deep infiltrative endometriosis (DIE). Accurate imaging is critical for surgical planning and improving patient outcomes (12, 13). (Figure 16)
Pelvic Inflammatory Disease (PID)
PID refers to an infection that spreads from the vagina or cervix to the upper genital tract, potentially causing infertility through tubal and peritubal damage.
MRI in PID
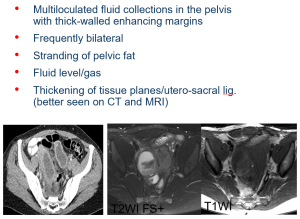
MRI is reserved for complex or unclear cases and can identify complications such as hydrosalpinx, pyosalpinx, or tubo-ovarian abscesses. (Figure 17)
Polycystic Ovary Syndrome (PCOS)
PCOS is a common endocrine disorder characterised by chronic anovulation and hyperandrogenism. Diagnosis is based on the Rotterdam criteria, which require the presence of at least two of the following features:
- Oligo- or anovulation
- Clinical or biochemical signs of hyperandrogenism
- Polycystic ovarian morphology on ultrasound or elevated serum AMH levels
MRI in PCOS
MRI findings in PCOS are nonspecific but can provide supportive evidence. Typically, ovaries in PCOS contain multiple small follicles arranged peripherally around a dense stromal core. (Figure 18)