Patients' population
This was an IRB-approved prospective study; informed consent was acquired. In the period between October 2024 and December 2024 we considered for inclusion all the patients who were scheduled for a prostate mpMRI on our 3T scanner (Siemens Magnetom Vida) at Bolzano Central Hospital. Inclusion criteria was the presence of at least one focal T2-hypointense lesion >4 mm in the peripheral zone. Exclusion criteria was insufficient image quality due to motion or metal artifacts.
MRI protocol
In the included patients, in addition to the standard protocol performed according to the PI-RADS v2.1 guidelines
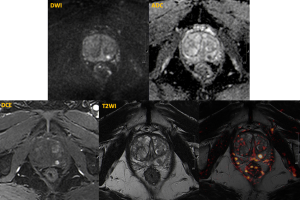
Image analysis
Image analysis was performed by 2 radiologists in consensus. For each lesion, the presence of focal early enhancement on DCE was evaluated and classified as either positive or negative. Additionally, each lesion was assigned a PI-RADS v2.1 category.
Prostate blood flow (PBF, measured in ml/100g/min) was quantified using the ASL technique by manually drawing regions of interest (ROIs) around each lesion and in the adjacent normal prostate tissue. 
The lesion-to-prostate PBF ratio was then calculated and compared to the DCE findings using the Mann-Whitney test.