Venous thromboembolism (DVT, PE) is a common disease that is on the minds of most physicians, has standardized management, and defined imaging diagnosis(1). However, non-thrombotic pulmonary embolism (NTPE) is a rare entity with an incidence that varies in studies depending on the cause.
NTPE is defined as the partial or total occlusion of pulmonary arterial vessels by non-thrombotic material, which can be of organic or inorganic type(2).
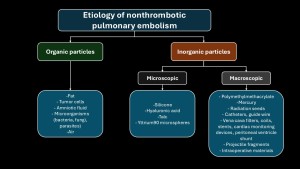
The pathophysiology of non-thrombotic pulmonary embolism is complex and depends on two factors(3–5):
- The size of the embolized particle (microscopic vs macroscopic).
- Type of embolized material: its interaction with pulmonary parenchyma.
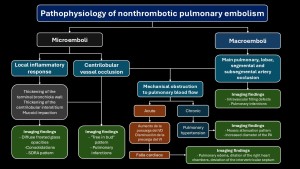
Symptoms range from asymptomatic to severe cases like acute respiratory failure. Clinical signs are nonspecific, and imaging plays a crucial role(4,5).
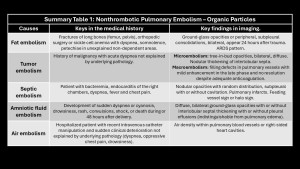
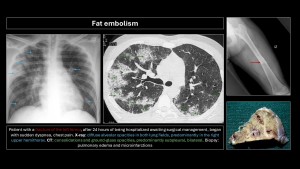
Fat embolism
Secondary to the rupture of fat tissue and its entry into systemic circulation, usually after fractures or bone surgery but also in acute pancreatitis, burns, liposuction, or sickle cell crises(6).
Prevalence is 1-3% in simple fractures of long bones; 20%-30% in polytraumatized patients with bilateral femoral or pelvic fractures(5).
Patients present with acute respiratory distress, altered consciousness, and petechiae in non-dependent areas in 85% of cases, but these disappear quickly and are not pathognomonic(5,6).
Main differential diagnosis: pulmonary contusions. They differ in the time of onset: contusions are immediate, unilateral, and resolve within 48 hours; opacities due to fat embolism appear 24-48 hours later, are bilateral, and take longer to resolve(5–9).
CT findings: Centrilobular nodules, ground-glass opacities, or bilateral, peripheral, subpleural consolidations predominating at the bases, which may progress to ARDS (microembolism)(5–10).
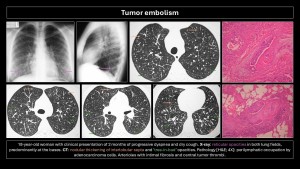
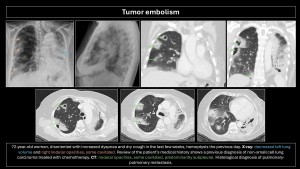
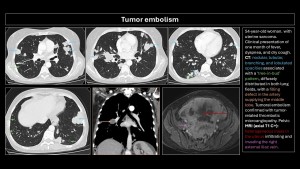
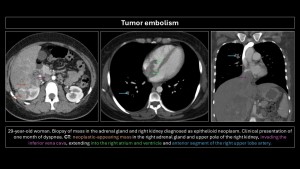
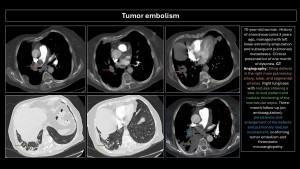
Tumor embolism
Described in 1937 by Brill and Robertson(11). It occurs when tumor cells lodge in pulmonary arteries, rarely invading the parenchyma. Incidence is difficult to evaluate; postmortem prevalence is 3-25%. Microembolism (gastric adenocarcinoma, breast, lung, pancreas, prostate) or macroembolism (renal carcinoma, sarcomas, choriocarcinoma, hepatocarcinoma, Wilms tumor, lymphoma) can occur(12,13).
In microembolism, tumor cells accumulate in blood vessels without invading pulmonary parenchyma, generating thrombotic microangiopathy (pulmonary parenchymal inflammation causing fibrocellular intimal hyperplasia and connective tissue proliferation)(3).
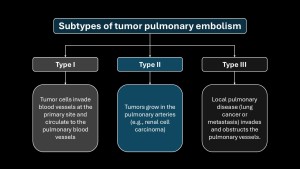
Subtypes of tumor embolism are shown in Figure4.
The main differential diagnosis is PE. Filling defects are similar and often indistinguishable on imaging. Cancer patients have a 4-fold higher risk of PE; a filling defect is more likely PE than a tumor embolus. Tumor microembolism manifests as “tree-in-bud” opacities and nodular thickening of interlobular septa(5,9,10,12).
Specific imaging signs of tumor embolism(2,8):
- Peripheral arteries dilated in a rosary-like pattern.
- Delayed enhancement of filling defects with contrast medium.
- Uptake of F18-fluorodeoxyglucose (FDG).
- No resolution of the filling defect in follow-up exams of patients receiving adequate anticoagulant therapy.
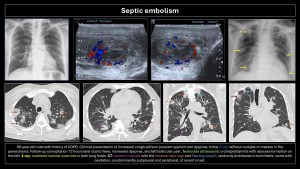
Septic embolism
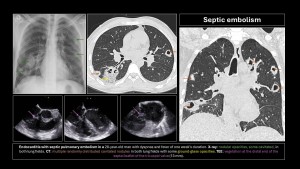
Occurs due to hematogenous dissemination of infected thrombi (bacteria, fungi, parasites) in the context of vegetations on catheters, right-sided endocarditis, septic thrombophlebitis, Lemierre’s syndrome, or osteomyelitis(2,3,5,7).
Clinical presentation: variable, with fever, dyspnea, chest pain, and even sepsis. Diagnosis is based on clinical suspicion, CT, echocardiography, and positive blood cultures.
Imaging findings:
- X-ray: Alveolar opacities with bilateral cavitary nodules.
- CT: Wedge-shaped opacities (pulmonary infarction) and multiple nodules of varying sizes, random distribution, subpleural location, which may or may not cavitate. Mediastinal lymphadenopathy with or without pleural effusion. The right heart cavities should be reviewed for vegetations. Frequent but nonspecific signs: “nutrient vessel sign” (visualization of the blood vessel leading to the nodule) and “halo sign” (central nodule with surrounding ground-glass opacity due to hemorrhage)(8,9).
Immunosuppressed patients with hematologic diseases may present with fungal embolism caused by Aspergillus, Mucor, or Candida, leading to vascular invasion, hemorrhage, thrombosis, and distal infarction.
Hydatid embolism is rare and manifests as cystic filling defects in pulmonary arterial vessels.
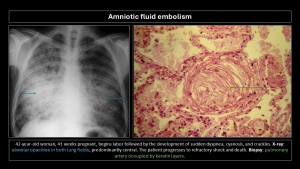
Amniotic fluid embolism
Presence of fetal squamous cells, mucin, meconium, vernix caseosa, or lanugo hairs in maternal pulmonary vessels.
It is one of the leading causes of maternal mortality in the developed world, accounting for 10% of all maternal deaths in the U.S., but its incidence is low, at 7.7 cases/100,000 live births(14).
Clinical presentation: sudden onset, acute respiratory distress, cyanosis, irritability, skin rash, seizures, and cardiogenic shock during delivery or within the first 48 hours. Amniotic fluid enters maternal circulation during delivery or, less frequently, during cesarean section through small tears in uterine veins, depositing in pulmonary circulation with flow obstruction and an immune reaction against amniotic fluid similar to anaphylaxis. Severity depends on the amount of embolized fluid and immune status(5-8). Maternal mortality rate is up to 80%, and fetal mortality rate is 40%(9,15).
Imaging findings:
- X-ray: Bilateral diffuse homogeneous opacities similar to pulmonary edema.
- CT: Bilateral diffuse ground-glass opacities with interlobular septal thickening, with or without pleural effusion, indistinguishable from other causes of pulmonary edema. Due to high mortality, most patients do not undergo CT.
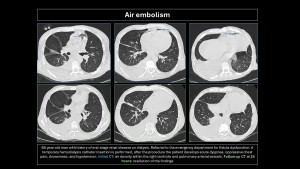
Air embolism
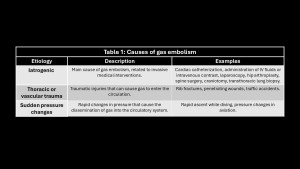
Air (oxygen, CO2, N2O, nitrogen, and helium) enters pulmonary circulation. The amount and rate of entry determine symptoms. The minimum lethal dose in humans is estimated at 300-500 mL(16,17). Causes are listed in Table1.
Clinical presentation: Sudden dyspnea, hypotension, chest pain, altered mental state, or seizures(16).
CT findings: Air density within blood vessels or right heart chambers; pulmonary edema may develop(3,5,9,10).
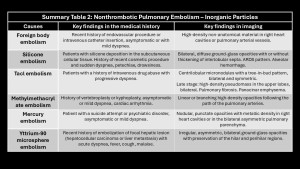
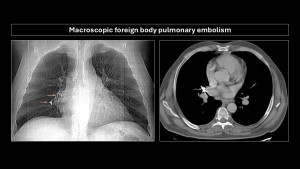
Macroscopic foreign body pulmonary embolism
Main embolized foreign bodies are listed in Table2.
CT is the imaging modality of choice when suspecting foreign body embolism, as it precisely localizes and aids in planning endovascular or surgical treatment.
Catheter tip embolism has an incidence of 1%-4%, with a mortality rate of 1.8%. Catheters can fragment during needle withdrawal, spontaneous rupture, or mechanical tension (pinch-off syndrome)(18).
The increase in intravascular procedures has led to more complications. Embolism can cause pulmonary vascular occlusion and arrhythmias if devices lodge in the right heart. Recommended treatment is endovascular retrieval; surgery may be indicated if it fails or the object is large(2,3,5,8,10).
A bullet fragment or entire projectile can enter the venous vascular system, though rare. Findings: mismatch between the number of entry and exit wounds, identifying the projectile in the lung without pulmonary contusion, soft tissue injury, or pneumothorax.
Silicone embolism
Liquid silicone (polydimethylsiloxane) is increasingly used for cosmetic augmentations. Complications occur with subcutaneous or intramuscular administration, causing local inflammation and vasodilation that facilitate migration to the venous capillary system and, subsequently, pulmonary arterial vessels, leading to flow obstruction and parenchymal inflammation(4).
Breast implants are covered with a capsule preventing embolization into pulmonary vessels, though cases of chronic embolism have been reported(7,10).
Clinical presentation: Similar to fat embolism, with acute dyspnea, petechial rash, altered mental status, and hypoxemia, appearing minutes after silicone injection.
Imaging findings: Bilateral, diffuse ground-glass opacities, predominating in lower lobes, with or without interlobular septal thickening, ARDS pattern and/or alveolar hemorrhage.

Talc embolism (hydrated magnesium silicate)
Oral medications contain excipients (talc, cellulose, starch) that, when dissolved for intravenous administration, remain insoluble and deposit in pulmonary vessels, causing obstruction and inflammation leading to granulomatous giant cell reaction and subsequent fibrosis(19).
Most patients are intravenous drug abusers (methadone, methylphenidate, etc.) presenting with progressive dyspnea(2,4).
CT findings: Centrilobular micronodules, “tree-in-bud” pattern, bilateral and symmetric, which may coalesce to form high-density masses and, in late stages, massive fibrosis or panacinar emphysema(2,4,9,10).
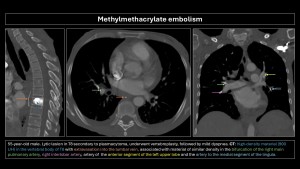
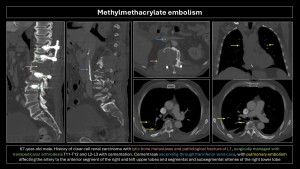
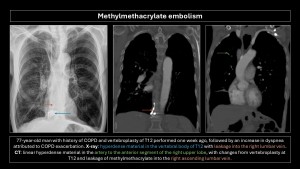
Methylmethacrylate embolism
Methylmethacrylate embolism is iatrogenic following vertebroplasty or kyphoplasty, occurring when injected methylmethacrylate extravasates into soft tissue, vertebral venous plexuses, and/or inferior vena cava, with an incidence of 30%-70%. Pulmonary embolism occurs in 3%-23% of cases(20). Risk factors include lower cement viscosity and absence of valves in the paravertebral venous plexus(2,4,5).
Imaging findings: Small, linear, high-density branching opacities following pulmonary artery trajectories; the bone window helps detect them(8–10).
Most patients are asymptomatic, but some may present vague symptoms, and a small proportion may develop pulmonary hypertension, arrhythmias, cardiac rupture, and death.
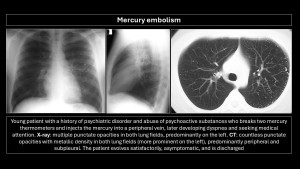
Mercury embolism
Intravascular injection of mercury is rare. Patients often have psychiatric disorders or suicide attempts. Prognosis is excellent.
Imaging findings: Multiple nodular, punctiform opacities with metallic density in the right heart chambers or diffusely scattered in the bilateral pulmonary parenchyma(2,9,10).
Summary