Introduction
Sarcoidosis is a multisystemic granulomatous disease of unknown etiology, characterized by the formation of non-caseating granulomas in various organs [1,2]. Although it can affect virtually any organ, pulmonary involvement and thoracic lymphadenopathy are predominant, with abnormalities observed on chest radiographs in more than 90% of patients with thoracic sarcoidosis [1,3]. In radiology, high-resolution computed tomography (HRCT) is superior to chest radiographs for assessing the extent of the disease [3,4]. Typical HRCT findings include micronodules with perilymphatic and broncho-centric distribution, perihilar opacities, and varying degrees of fibrosis [4,5]. These findings can help differentiate between active inflammatory components, which may respond to treatment, and irreversible fibrosis, for which treatment is not indicated [3,6]. Additionally, positron emission tomography (PET/CT) with fluorodeoxyglucose (FDG) is useful for assessing the inflammatory activity of sarcoidosis in any organ, providing valuable information on the extent of the disease and aiding in diagnosis and management [4,7]. In summary, radiology plays a crucial role in the diagnosis and management of sarcoidosis, with HRCT and PET/CT being essential tools for evaluating the extent and activity of the disease, as well as distinguishing between active inflammation and fibrosis [2,5].
General Radiological Features
Sarcoidosis exhibits diverse radiological manifestations depending on the imaging modality and the affected organ system [3,5]. The imaging findings are typically characterized by the presence of non-caseating granulomas and associated inflammatory changes. The following are some of the general radiological characteristics observed across various imaging modalities [3,4,8].
Radiography
Chest radiographs are often the first-line imaging modality, showing abnormalities in more than 90% of patients with thoracic sarcoidosis [1,3]. Common findings include bilateral hilar lymphadenopathies, often symmetrical, reticulonodular opacities with a perihilar and upper lobe predominance, and advanced stages may reveal signs of pulmonary fibrosis with architectural distortion and volume loss [1,4].
Computed Tomography (CT)
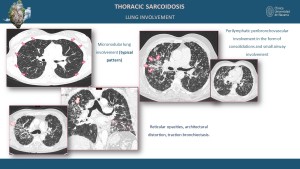
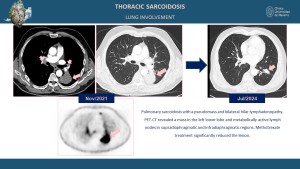
High-resolution computed tomography (HRCT) provides superior detail for pulmonary involvement, often revealing perilymphatic micronodules along the bronchovascular bundles, interlobular septa, and pleura, ground-glass opacities and consolidations indicating active inflammation, and fibrotic changes such as honeycombing, traction bronchiectasis, and architectural distortion in chronic stages [3,5,7].
Magnetic Resonance Imaging (MRI)
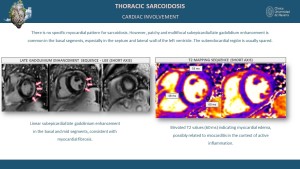
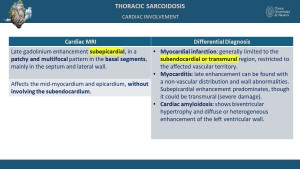
Cardiac sarcoidosis can be evaluated using computed tomography (CT) and magnetic resonance imaging (MRI), with modality-specific findings [6,9]. CT typically shows nonspecific features such as myocardial thinning, cardiomegaly, pericardial effusions, and ventricular aneurysms [3,6]. MRI, however, is more sensitive for detecting cardiac involvement, often revealing late gadolinium enhancement (LGE) in the mid-myocardium and/or epicardium, particularly in the basal regions of the heart, such as the septum and lateral wall, while sparing the subendocardium [6,9]. Additionally, MRI may demonstrate hyperintense nodular foci on T2-weighted sequences and areas of focal myocardial thickening [9,10].
Positron Emission Tomography (PET/CT)
PET/CT with fluorodeoxyglucose (FDG) is also a highly sensitive imaging technique, particularly for detecting active inflammation [7,11]. Findings include increased FDG uptake in affected lymph nodes and organs, which is useful for evaluating systemic involvement and guiding biopsy [5,7].
Organ-Specific Radiological Features
Thoracic Involvement
Bilateral hilar and mediastinal lymphadenopathy is the hallmark feature, and HRCT may show lymph nodes with homogeneous enhancement or calcification in chronic cases [1,3]. Pulmonary parenchymal disease often exhibits a perilymphatic distribution of micronodules, predominantly in the upper and middle lung zones, and fibrotic changes in advanced disease, including traction bronchiectasis and conglomerate masses [3,5].



Cardiac Involvement
Cardiac sarcoidosis is observed in up to 25% of patients, often presenting subclinically [6,10]. MRI findings typically include patchy late gadolinium enhancement (LGE) in a non-vascular distribution, predominantly involving the mid-myocardium and/or epicardium, particularly in the basal regions such as the septum and lateral wall, while sparing the subendocardium [9]. Additional MRI features may include focal myocardial thickening, hyperintense nodular foci on T2-weighted sequences indicative of edema, wall motion abnormalities, and regions of myocardial thinning [6,9]. PET/CT commonly reveals focal or diffuse FDG uptake in the myocardium, indicative of active inflammation [7,11]. CT, while less specific, may demonstrate findings such as myocardial thinning, cardiomegaly, pericardial effusion, or ventricular aneurysms [6,10].
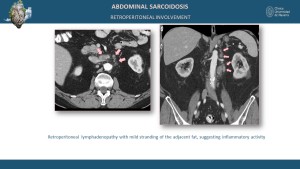
Abdominal Involvement
In the liver and spleen, findings include hepatosplenomegaly with diffuse or focal hypodense lesions on CT and T2 hyperintense lesions with variable contrast enhancement on MRI [1,4]. Kidney involvement may present as cortical nodules or mass-like lesions with hypodense areas on CT and hypo- to isointense signals on MRI [8,11].
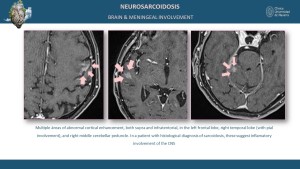
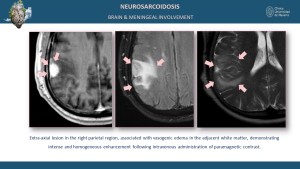
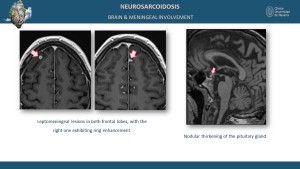
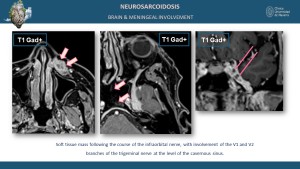
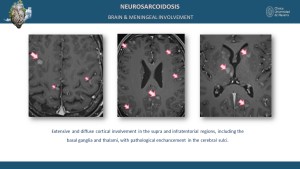
Neurological and Meningeal Involvement
Neurological sarcoidosis occurs in 5-15% of cases, involving the cranial nerves, brain parenchyma, meninges, and spinal cord [5,9]. MRI findings include leptomeningeal enhancement on post-contrast sequences and parenchymal T2 and T2 FLAIR hyperintensities in the brain or spinal cord [8].

Spinal Cord Involvement
Spinal cord involvement is characterized by focal or diffuse spinal cord enlargement and T2 hyperintense lesions with post-contrast enhancement [8,12].

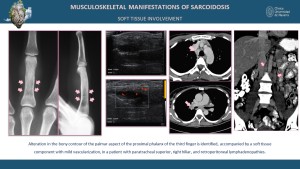
Musculoskeletal and Soft Tissue Involvement
Soft tissue involvement in sarcoidosis may present as nodular or mass-like lesions, often with homogeneous or heterogeneous enhancement observed on CT and MRI [3,5,11]. Bone involvement typically manifests as lytic or sclerotic lesions, frequently affecting the small bones of the hands and feet [8,12]. These changes often present a lace-like or reticulated pattern of bone resorption, which is characteristic of sarcoidosis [12]. Advanced imaging modalities, such as CT and MRI, are instrumental in detecting these findings and differentiating them from other pathologies [3,8]. Small bone involvement, particularly in the phalanges, may lead to cortical thinning, trabecular bone loss, and deformities such as pathological fractures, as described in the literature [12]. These distinct imaging features provide critical diagnostic clues for early identification and management of the disease [4,8,12].
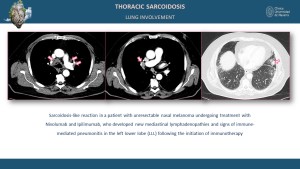
Sarcoidosis-like Reactions Induced by Immunotherapy
Sarcoidosis-like reactions have been increasingly reported in patients undergoing immunotherapy, particularly immune checkpoint inhibitors (ICIs) such as anti-PD-1, anti-PD-L1, and anti-CTLA-4 antibodies [11]. These reactions are thought to result from enhanced immune activation leading to granuloma formation [7]. Radiologically, these reactions may mimic sarcoidosis, with findings such as bilateral hilar lymphadenopathy, perilymphatic micronodules, and FDG-avid lymph nodes and organs on PET/CT [11]. Differentiating between true disease progression and immunotherapy-induced sarcoidosis-like reactions is crucial, as the latter often resolves with immunotherapy cessation or corticosteroid treatment [7,11].
Management
The management of sarcoidosis necessitates a multidisciplinary approach, with imaging playing a pivotal role in guiding treatment strategies [3,4]. High-resolution CT (HRCT) is instrumental in evaluating pulmonary involvement, detecting parenchymal abnormalities, and assessing disease progression [1,3,5]. MRI is particularly valuable for assessing cardiac and neurological sarcoidosis, providing detailed tissue characterization and identifying active inflammation [6,9]. PET/CT, especially with fluorodeoxyglucose (FDG), is effective in detecting active inflammatory sites and assessing the extent of systemic involvement [4,7]. These imaging modalities are essential for monitoring treatment response, detecting complications, and guiding biopsy procedures to obtain histopathological confirmation [4,6,9].