Findings and procedure details
The following key factors predispose cancer patients to infections:
Disruption of natural barrier
- Damage to the skin, the protective lining of the gastrointestinal (GI) tract, or other mucosal tissues in the abdomen or pelvis allows pathogen translocation, leading to concurrent infection. Injury is usually secondary to cancer treatments like chemotherapy, radiation, or immunotherapy, but can also occur through direct tumor invasion (for example, gastrointestinal ulcerative tumors). Mucosal barrier disruption occurs in four phases: the inflammatory/vascular phase, the epithelial/apoptosis phase (which coincides with neutropenia), the necrosis and ulceration phase, and the healing phase [5].
Immunosuppression, neutropenia
- Immunosuppression can result from both the malignancy itself and the treatments used to combat it. While hematologic malignancies directly affect the immune system by infiltrating the bone marrow or other immune tissues, solid tumors compromise immunity by releasing immunosuppressive factors. A frequent consequence of chemotherapy is neutropenia (neutrophil count of <1500 cells/mm³). Patients with hematological malignancies are most predisposed to multidrug-resistant infections due to their prolonged periods of febrile neutropenia [6].
Tumor obstruction
- Solid cancers, especially in the genitourinary, colorectal, and hepato-pancreatobiliary systems, often cause obstruction, leading to post-obstructive infections, perforations, or abscesses. Advanced tumors in these areas are particularly prone to secondary infections.[7,8].
Vascular occlusion
- Vascular occlusion can occur due to a thrombus in hypercoagulable states, direct tumor extension to vessels, or mass effect on underlying vascular structures (in cases of solid tumors). Reduced blood flow and tissue damage caused by vascular blockage facilitate infection. Cancers associated with a high risk of thromboembolic events include malignancies of the pancreas, uterus, ovaries, stomach, and kidneys [9].
Treatment related
- Treatments like chemotherapy, radiation, immunotherapy, and corticosteroids can weaken the immune system by targeting healthy cells or limiting the immune response. Acute radiation primarily causes inflammation of the mucosal barrier, while chronic radiation leads to complications like transmural fibrosis, vascular sclerosis, or fistulas with abscess formation [10]. Anastomotic bowel leaks or intra-abdominal infections are feared complications after surgery for colorectal cancer [11]. Catheters and devices (e.g., gastrostomy tubes, biliary/urinary stents) can be complicated by infection, either by harboring pathogens or causing direct obstruction.
HEPATOBILIARY INFECTIONS
Cholangitis is a frequent complication of biliary tract obstruction with stasis caused by hepato-biliary, pancreatic, or duodenal tumors, with or without the formation of liver abscesses.
Fig 1: Acute cholangitis and biliary obstruction in a 48-year-old female with metastatic cancer. (A) Axial and (B) coronal portal venous-phase images show confluent retroperitoneal lymphadenopathy (asterisks) causing obstruction of the common hepatic duct with intrahepatic biliary ductal dilatation (arrowheads). Focal circumferential enhancement of bile duct walls (arrows) suggestive of cholangitis can be detected. [Itani, M., et al. (2021). Imaging of abdominal and pelvic infections in the cancer patient. Abdominal Radiology, 46, 2920-2941.]
Fig 2: Recurrent pyogenic cholangitis in a 56-year-old female with cholangiocarcinoma. Initial (A) contrast-enhanced CT scan shows a large hypodense mass in the right lobe with peripheral enhancement (arrows), later confirmed as an abscess. Atrophy of the left lobe with inhomogeneous enhancement and intrahepatic biliary ductal dilatation is also observed. Images obtained five months later (B) show abscess resolution, extensive atrophy, and a new mass in the left lobe, confirmed as cholangiocarcinoma. [Kim, J. H., et al. (2006). CT findings of cholangiocarcinoma associated with recurrent pyogenic cholangitis. American Journal of Roentgenology, 187(6), 1571-1577]
Fig 3: Liver abscess in an 83-year-old female with cholangiocarcinoma. Emergency non-enhanced CT scan (A) shows a hypodense round lesion in the right lobe (green arrow). CT images obtained three days later (B-D) show an increase in size of the previous lesion with peripheral enhancement, suggesting an abscess. Atrophy of the left lobe and a lesion with indistinct margins and inhomogeneous peripheral and central enhancement are also observed (green circle). There is significant dilatation of the biliary ducts in both lobes (white arrows). MRI images better characterize the lesions, indicating biliary obstruction and a liver abscess associated with left lobe peripheral cholangiocarcinoma. [Department of Radiology and Medical Imaging, Brasov County Emergency Clinical Hospital, 2024/ Romania]
Fig 4: Hepatic and appendiceal mucormycosis in a 41-year-old female with acute T-cell lymphoblastic leukemia. During the neutropenic phase post-chemotherapy, an urgent CT was obtained. (A) Coronal image shows extensive non-enhancing hypodense lesions in the liver, as well as mural thickening and enhancement of the appendix with adjacent fat infiltration (arrow). Antifungal therapy was started, and two weeks later, follow-up CT and MRI images show (B) clearer margins with peripheral rim-like enhancement (arrowheads) of the liver and appendiceal lesions. Axial MRI images show (C) internal T2 high signal with (E) progressive peripheral enhancement, correlated with high b-value on (F) diffusion-weighted imaging. [Choi, S. Y., et al. (2015). CT and MR imaging findings of appendiceal and hepatic mucormycosis in a patient with acute T-lymphoblastic leukemia. Journal of the Korean Society of Radiology, 73(5), 328-332.]
Fig 5: Clostridium-infected liver metastases in a 61-year-old patient with colonic cancer. CT (A) axial and (B) coronal images show pneumoperitoneum (green arrows) and multiple liver masses containing gas (white arrows), raising suspicion for abscesses. Emergency right hemicolectomy was performed, later confirming intestinal adenocarcinoma. Biopsy established the metastatic nature of the liver masses. [Vázquez-Melero, A., et al. (2017). Pneumoperitoneum and gas-forming liver abscesses as initial signs of colorectal liver metastases. Surgical Infections Case Reports, 2(1), 85-87.]
Fig 6: Hepato-spleno-renal candidiasis in a 26-year-old male with AML and severe neutropenia. Ultrasound (A) transverse and (B) longitudinal images show lesions with an echogenic center (inflammation) and a peripheral hypoechoic ring (fibrosis), exhibiting the typical ‘bull’s eye’ appearance. Axial (C, D) contrast-enhanced CT images show multiple hypodense subcentimeter abscesses in the liver, spleen, and kidneys (arrows). [Itani, M., et al. (2021). Imaging of abdominal and pelvic infections in the cancer patient. Abdominal Radiology, 46, 2920-2941.]
Surgical procedures increase the risk of infection, as observed after the administration of intra-arterial chemotherapy for hepatocellular carcinoma or after liver biopsy, which can lead to secondary liver abscesses or cholangitis resulting from internal biliary drainage and pathogen colonization of stents [13].
Fig 7: Liver abscess after TACE (transarterial chemoembolization) with lipiodol for HCC in a 72-year-old male. (A) CT at diagnosis shows a well-circumscribed mass with enhancement in the arterial phase in the 6th segment of the liver. (B) After the TACE procedure, complete accumulation of lipiodol in the HCC is seen. One month later, the patient presented to the emergency room. (C,D) Axial CT images show an ill-defined mass with multiple gas bubbles located superior to and in contact with the treated HCC, suggestive of an abscess. [Yasutomi, E., et al. (2021). Liver abscess caused by Cutibacterium namnetense after transarterial chemoembolization for hepatocellular carcinoma. Clinical Journal of Gastroenterology, 14, 246-250.]
Fig 8: Acute adenovirus hepatitis in a 49-year-old male with diffuse large B cell lymphoma and a bone marrow transplant. (A) Axial T2-weighted image shows a subcapsular wedge-shaped area with mild hyperintense signal (arrowhead). T1-weighted images obtained in (B) arterial, (C) equilibrium, and (D) 5-minute delayed phases demonstrate no arterial enhancement, with progressive equilibrium and delayed fill-in, and no mass effect or vascular displacement. Liver biopsy confirmed the inflammatory etiology. [Itani, M., et al. (2021). Imaging of abdominal and pelvic infections in the cancer patient. Abdominal Radiology, 46, 2920-2941.]
GASTROINTESTINAL TRACT INFECTIONS
Clostridioides difficile colitis (formerly Clostridium difficile) is the most common healthcare-associated infection and is twice as likely to occur in cancer patients a few weeks after antibiotic therapy. C. difficile colitis can range from mild to fulminant, with the latter characterized by hypotension/shock, ileus, and/or megacolon (> 6 cm), with potential bowel perforation.
Fig 9: Clostridium difficile pseudomembranous colitis in a 62-year-old male with large B cell lymphoma. (A) Axial and (B) coronal contrast-enhanced CT images show marked pancolitis, manifested by wall thickening, submucosal edema, mucosal hyperenhancement (‘accordion sign’), pericolonic fat stranding, and reactive ascites (asterisk). Shedding of the mucosa (arrowheads) results in the pseudomembranous appearance. [Itani, M., et al. (2021). Imaging of abdominal and pelvic infections in the cancer patient. Abdominal Radiology, 46, 2920-2941.]
Neutropenic enterocolitis (typhlitis) is commonly encountered in hematologic malignancies following cytotoxic chemotherapy, particularly in patients with preexisting bowel pathology, such as diverticulitis, tumor infiltration, or previous surgery. The infection is usually polymicrobial and typically occurs in the third week after treatment when the mucosa is most damaged and neutropenia is significant [14].
Fig 10: Neutropenic enterocolitis in a patient with acute myeloid leukemia. (A,B) Ultrasound transverse plane images show severe bowel wall thickening of the cecum, with clear stratification of the wall layers: the hypoechoic virtual lumen and mucosa (white arrowheads), the wide hyperechoic submucosa (black arrows), and the hypoechoic peripheral muscularis mucosa (black arrowheads). (C) Longitudinal plane image shows a longer segment of bowel involvement. [Pugliese, N., et al. (2017). Ultrasonography‐driven combination antibiotic therapy with tigecycline significantly increases survival among patients with neutropenic enterocolitis following cytarabine‐containing chemotherapy for the remission induction of acute myeloid leukemia. Cancer Medicine, 6(7), 1500-1511.]
Fig 11: Neutropenic colitis in two patients with hematological malignancies. (A) CT coronal reconstructed image of a 45-year-old male with acute B cell lymphoblastic leukemia shows diffuse colonic wall thickening with submucosal edema and mucosal hyperenhancement, affecting the cecum, ascending, transverse, and the majority of the descending colon (black arrows). Thickening of the parietal peritoneum, fat stranding, and ascites are also observed (white arrows). (B) CT coronal reconstructed image of a 53-year-old male with acute myeloid leukemia shows parietal thickening with submucosal hypodensity, affecting only the cecum (black arrows). The remainder of the bowel is unremarkable (white arrowheads), and there is no free fluid. [Case courtesy of Sim K Typhlitis, Radiopaedia.org, rID: 33256] [Case courtesy of Di Muzio B, Radiopedia.org, rID:43967]
Gastrointestinal fungal infections are common in cancer patients, which is why antifungal therapy is often introduced, particularly in patients with hematologic tumors. Aspergillus, Candida, and Mucorales are the most commonly involved species affecting the gastrointestinal tract, as well as solid organs, peritoneum, and/or abdominal wall. Both Mucorales and Aspergillus infections can cause large ulcers with irregular edges due to their angioinvasive nature, and are associated with high mortality [15].
Viral gastrointestinal infections are less common but carry a high degree of mortality, especially Cytomegalovirus (CMV), which affects the colon, stomach, and esophagus. CMV infection, associated with graft-versus-host disease (GVHD), is an important complication after allogeneic hematopoietic stem cell transplantation (HSCT). Norovirus is also a highly contagious virus that can lead to severe and complicated gastroenteritis in immunosuppressed patients.
Perianal and perirectal infections are commonly encountered in neutropenic patients or those with genitourinary or anorectal solid tumors, with neoadjuvant radiation being a major risk factor.
Fig 12: Rectovesical fistula in a 60-year-old male with rectal adenocarcinoma. (A) Axial venous-phase image shows tumoral wall thickening of the rectum (arrowheads) extending to the bladder wall (black arrow). The presence of gas within the bladder lumen raises suspicion for a fistula, which is confirmed on the delayed-phase images (B,C), with contrast visible at the communication site (green arrow). [Case courtesy of Niknejad M, Radiopaedia.org, rID: 21274]
Fig 13: Radiation-induced proctitis in a 78-year-old female with primary vaginal cancer. (A) Coronal T2-weighted image shows significant thickening of the rectal (green arrow) and sigmoid walls (white arrowheads). (B-C) Axial T2-weighted images demonstrate a residual mass involving the right upper aspect of the vagina, touching the bladder floor (black arrow). [Case courtesy of Di Muzio B, Radiopaedia.org, rID: 31658]
Fig 14: Small bowel obstruction secondary to radiation-induced enteritis in a 40-year-old female with cervical cancer. (A-C) Contrast-enhanced CT images show multiple loops of dilated small bowel (white arrows) with a transition point (circle) into a segment of mildly thickened small bowel in the pelvis (arrowheads), suggesting radiation-related enteritis. Small ascites is also present (green arrows). [Case courtesy of Hartung M , Radiopaedia.org, rID: 154832]
Fig 15: Rectouterine fistula and rectal bleeding secondary to radiation proctitis in a 72-year-old female with previous cervical carcinoma. (A) Axial and (B) coronal reformatted MIP images show diffuse mural thickening involving the entire rectum (arrows), associated with mild mesorectal fascial thickening and fat stranding, suggesting chronic radiation proctitis. Active extravasation of contrast material from the middle rectal artery into the rectum is observed (asterisk). (C) Sagittal reformatted non-enhanced CT demonstrates a large communication between the rectum and cervix, with feculent material in the vagina (arrows). [Pasupuleti J, et al. (2020).Imaging Findings in LateOnset Rectal and Vesical Bleed in Patients Who Have Undergone Radiation Therapy for Pelvic Neoplasms. Asian J. Med. Radiol. Res.;8(2):61-64]
Fig 16: Radiation enterocolitis with small bowel and sigmoid perforation and entero-colonic fistula in a 66-year-old female with previous cervical cancer. (A,B) Emergency contrast-enhanced CT scan shows free air in the peritoneum (white arrows), small bowel thickening with mucosal enhancement, and multiple inflammatory adhesions with a possible connection between the small bowel and sigmoid colon (arrowheads). Free peritoneal fluid and mesenteric edema are also seen. Exploratory laparotomy confirmed the entero-colonic fistula associated with a 1 cm perforation. [Meng M, et al.(2023) Radiation Enterocolitis Featuring the Perforation of the Sigmoid Colon, Small Bowel, and Entero-Colonic Fistula: A Case Report. Cureus 15(8): e43167]
GENITOURINARY INFECTIONS
Pyelonephritis and pyonephritis are complications of urinary tract obstruction with stasis or pathogen colonization of pigtail ureteral stents or nephrostomy diversion tubes.
Fig 17: Pyonephrosis complicated by liver abscess in a 53-year-old male with a previous cystectomy for papillary transitional cell carcinoma. (A,B) Non-enhanced CT images show an enlarged kidney (20 cm) with significant dilatation of the collecting system containing fluid with higher attenuation values (mean 18 HU) and gas-fluid levels. Marked fat stranding and thickening of the pararenal fascia are present (arrowheads). (C-E) A percutaneous nephrostomy tube was inserted for drainage, and a follow-up CT performed 10 days later showed partial resorption of the collecting system collections, with persistence of an upper pole collection that communicates with a collection containing fluid and gas in the posterior sector of the liver (green arrow). [Department of Radiology and Medical Imaging, Brasov County Emergency Clinical Hospital, 2024/ Romania]
Fig 18: Acute pyelonephritis after stenting procedure. CT portal-phase (A) axial, (B) coronal, and (C) curved MPR images show multifocal wedge-shaped areas of streaky enhancement in the middle and inferior regions of the right kidney (circle). The swirl of the stent is clearly visible in the pelvis (arrow). [Corvino, A., et al. (2024). Complications Subsequent to Urinary Tract Stent Placement: An Overview Focusing on the Imaging of Cancer Patients. Medicina, 60(2), 338.]
Fig 20: Kidney and prostatic abscesses in a 68-year-old male undergoing monoclonal antibody therapy for chronic lymphocytic leukemia. After 8 weeks of immunotherapy, a contrast-enhanced CT was performed. (A,B) Axial images show a relatively well-defined low-attenuation mass with an irregular wall in the right kidney and multiple well-defined hypodense lesions in the prostate, with inhomogeneous enhancement of the prostatic parenchyma. Prostate biopsy confirmed the fungal etiology. [Roux, C., et al. (2013). Prostatic and renal aspergillosis due to Aspergillus fumigatus in a patient receiving alemtuzumab for chronic lymphocytic leukemia. Journal de mycologie medicale, 23(4), 270-273.]
Fig 19: Emphysematous pyelonephritis with E. coli in a 92-year-old male with urothelial carcinoma of the bladder. (A,B) Axial and coronal reformatted non-enhanced CT images show air in the renal parenchyma, collecting system, and proximal ureter, with perirenal fat stranding. The left ureteral stent is displaced (not shown). (C) After stent replacement and antibiotic therapy, a follow-up CT performed 5 days later demonstrates significant improvement. [Singh, K., Greene, J. (2024). Emphysematous Pyelonephritis in Cancer Patients: A Case Report With a Literature Review. Cureus, 16(7), e65000.]
Ureteral obstruction is most often secondary to primary ureteral malignancy or other pelvic solid cancers, as well as compression by retroperitoneal adenopathies or treatment-induced strictures post-radiation therapy. Long-term use of Foley or suprapubic catheters and intravesical BCG for bladder cancer are predisposing factors for infectious cystitis.
Fig 21: Renal granulomas and granulomatous cystitis in a 58-year-old male after intravesical Bacille Calmette-Guérin treatment for bladder cancer. (A-C) Axial contrast-enhanced CT images show enhancing masses in the right kidney (black arrows) and thickening of the right bladder wall (white arrow). Bladder biopsy confirmed the granulomatous etiology, and appropriate treatment was initiated. (D,E) Axial contrast-enhanced CT images obtained 6 months later show a reduction in the size of the renal masses and bladder wall thickening. [Ma, W., et al.(2009). Imaging appearance of granulomatous disease after intravesical Bacille Calmette-Guerin (BCG) treatment of bladder carcinoma. American Journal of Roentgenology, 192(6), 1494-1500.]
Acute bacterial prostatitis and prostatic abscesses are most often caused by E. coli following medical procedures such as prostate biopsy, brachytherapy, or cryotherapy [16]. Gynecological infections are mainly caused by microorganism invasion due to obstructive processes or tumor necrosis.
Fig 22: Uterine carcinosarcoma presenting as polymicrobial sepsis in a 55-year-old female. Transvaginal ultrasound images show (A) endometrial thickening and masses consistent with fibroids. Contrast-enhanced CT with multiplanar reconstructions (B-D) reveals a fibroid uterus with an 8 cm expanded endometrial cavity and hyperenhancement within the mass (arrow). Deterioration of the clinical status led to surgical intervention with total hysterectomy, and pathology revealed an infected carcinosarcoma with Clostridium and Bacteroides. [Imo, C. S., et al. (2022). Clostridium and Bacteroides bacteremia as initial presentation of uterine carcinosarcoma. Gynecologic Oncology Reports, 42.]
Fig 23: Superinfection of a postsurgical lymphocele in a 41-year-old female with a history of radical hysterectomy and pelvic lymph node dissection for endometrioid ovarian carcinoma. (A-D) Axial and coronal contrast-enhanced CT images show a bilobated fluid-like collection with a thickened enhancing wall (arrows) adjacent to a metallic surgical clip (site of previously dissected obturator lymph node). (E) The right obturator internus muscle appears thickened with inhomogeneous enhancement (asterisk), suggesting contiguous spread of infection. [Case courtesy of Tonolini Massimo, Eurorad.org, case 11670]
Fig 24: Vesicovaginal fistula in a 66-year-old female with a history of radical hysterectomy, radiotherapy for endometrial carcinoma, and repeated surgery for left-sided pelvic neoplastic recurrence. (A) Initial contrast-enhanced CT shows a left-sided pelvic mass with predominant peripheral enhancement, suggesting tumor relapse (arrow). Postoperatively, (B) venous-phase coronal image demonstrates mass resection with surgical clips (arrowhead) and a left ureteral stent. (C-F) Axial and coronal excretory-phase images show bladder opacification and a laterally retracted, opacified vagina (arrows), with a short fistula (arrowhead). [Case courtesy of Tonolini Massimo, Eurorad.org, case 12239]
PERITONEAL, RETROPERITONEAL AND ABDOMINAL WALL INFECTIONS
Peritonitis is a serious complication in cancer patients, often leading to sepsis. Causes include bowel or stomach obstruction, perforation, or dehiscence of bowel or bilioenteric anastomosis. Iatrogenic causes include surgery, non-tunneled catheters, long-term peritoneal dialysis, and Bevacizumab/Sunitinib therapy [17].
Fig 26: Intestinal complications secondary to molecular targeted therapy in an asymptomatic 68-year-old man with renal cell carcinoma. (A) Axial lung window image from routine restaging CT shows extensive pneumatosis (arrow) involving the small bowel with a small pneumoperitoneum (arrowheads). Sunitinib was discontinued, and the findings were absent on the (B) follow-up CT 2 months later. [Shinagare, A. B., et al. (2012). Pneumatosis intestinalis and bowel perforation associated with molecular targeted therapy: an emerging problem and the role of radiologists in its management. American Journal of Roentgenology, 199(6), 1259-1265.]
Fig 25: CMV peritonitis following hematopoietic stem cell transplantation in a 59-year-old man with a history of acute myeloid leukemia. (A) Axial and (B) coronal reformatted CT images show diffuse fat infiltration of the omentum and mesentery, with enhanced nodular lesions along the right paracolic gutter, and no evidence of bowel perforation. Biopsy of the nodular peritoneal lesions confirmed the viral etiology. [Park, S. K. (2013). Primary Cytomegalovirus Peritonitis Following Unrelated Hematopoietic Stem Cell Transplantation. Soonchunhyang Medical Science, 19(2), 128-132.]
Peritoneal devices used for refractory ascites, dialysis catheters, ventriculoperitoneal shunts, or surgical drains can all be potential sites of infection due to translocation of skin flora, with or without the formation of abscesses.
Abdominal abscesses are usually of polymicrobial etiology and can be either peritoneal, secondary to peritonitis and/or perforation, or retroperitoneal, caused by visceral perforation or local spread of infection.
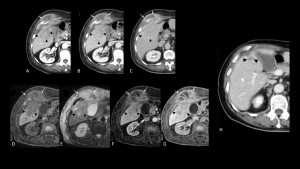
Fig 28: Actinomycosis liver abscess extending to the abdominal wall in a 73-year-old male with intrahepatic cholangiocarcinoma. Axial (A) arterial, (B) venous, and (C) delayed-phase CT images show a mass with predominantly peripheral enhancement (white arrows) in the right liver lobe, continuous with the abdominal wall. Another mass with irregular borders and delayed-phase marginal enhancement is present in the 5th segment. On MRI, the mass in the 5th segment shows low attenuation on (D) T1wFS and mild hyperintensity on (E) T2wFS, with similar (F,G) contrast enhancement as on CT, raising suspicion for cholangiocarcinoma with abdominal wall invasion. Biopsy confirmed the final diagnosis of cholangiocarcinoma with associated abdominal wall abscess. After antibiotic therapy and several rounds of chemotherapy, (H) follow-up CT showed complete resorption of the wall abscess and significant tumor shrinkage. [Masuda, T., et al. (2023). Intrahepatic cholangiocarcinoma with a liver abscess due to hepatic actinomycosis. Surgical Case Reports, 9(1), 43.]
Fig 29: Retroperitoneal conglomerate of lymph nodes complicated by abscess formation and small bowel fistula in a 49-year-old male with a history of left testicular neoplasm. Contrast-enhanced (A) axial, (B,C) coronal, and (D) sagittal CT images show a voluminous retroperitoneal paraaortic conglomerate of necrotic lymph nodes with peripheral enhancement (white arrows) and air-fluid levels, suggesting abscess formation. In the left flank, there is continuity between the mass and a segment of small bowel (circle), raising suspicion of fistula formation, prompting a CT with oral contrast the following day. (E-G) An 11 mm communication was confirmed between the proximal jejunum and the abscessed conglomerate of lymph nodes (green arrow), with inhomogeneous contrast filling of the abscess (arrowheads). [Department of Radiology and Medical Imaging, Brasov County Emergency Clinical Hospital, 2024/ Romania]
Skin and soft-tissue infections (SSTIs) in the abdominal wall include cutaneous and subcutaneous infections, fascial and muscular infections, and are most frequently encountered in patients with endarteritis obliterans caused by radiation therapy. Examples of SSTIs include cellulitis, necrotizing fasciitis, pyomyositis, as well as enterocutaneous or vesicocutaneous fistulas.
Fig 27: Postsurgical necrotizing fasciitis in a 78-year-old male with rectal neoplasm. Twenty-four hours after rectal surgery, a CT scan was performed due to worsening of the patient's condition. (A-D) Axial CT images show significant emphysema and edema extending along the right soft tissues, affecting the parietal abdominal muscles, which appear swollen with hypodense areas, and involving the subcutaneous fascia, which appears enhanced. The infection’s origin is traced to a right drainage pipe, as seen on the (E) coronal CT image. [Carbonetti, F., et al. (2014). A case of postsurgical necrotizing fasciitis invading the rectus abdominis muscle and review of the literature. Case Reports in Medicine, 2014(1), 479057.]




























