Diffusion-Weighted Imaging
In patients with varicocele, the malfunction of valves in the internal spermatic veins can raise pressure levels up to eight times higher than normal physiological levels, leading to testicular hypoxia and oxidative stress (OS) [8]. In addition to ischemic processes, fibrotic changes may also be linked to increased pressure and fluid accumulation in testes with varicocele [9-11]. All of these changes at the microscopic level are reflected in MRI, as significant differences in testicular ADC have been observed between patients with varicocele and healthy volunteers [1-4]. Specifically, the ADC of the ipsilateral testis in men with varicocele was lower than that of healthy individuals. Interestingly, the same study reported also a decrease in ADC of the contralateral testis in men with varicocele compared to controls [1]. Bilateral testicular biopsies in men with unilateral left varicocele report the presence of histologic alterations, such as maturation arrest and decreased spermatogenesis, sloughing of spermatogenic epithelium, Leydig cell hyperplasia, thickening of tubular basement membranes and interstitial blood vessel walls, and increased deposition of interstitial fibrous tissue in both testes [9-11]. Therefore, decrease in ADC of the contralateral testis in patients with varicocele is also related to hypoxia and fibrosis.
The above findings were reinforced by a more recent study, which confirmed a negative correlation between ADC and the diameter of the plexus pampiniformis veins. Additionally, the authors established a positive correlation between ADC and both sperm count and morphology, indicating that as ADC rises, both sperm count and quality improve. A cutoff of ADC 1.131 × 10−3 s/mm2 was found for the diagnosis of oligospermia (sensitivity: 94.3%; and, specificity: 87.5%), and a cutoff value of 1.25 × 10−3 s/mm2 for the detection of impaired sperm morphology (sensitivity: 86.6%; and, specificity: 43.8%) [3]. A significant positive correlation between lower ADC and low sperm motility has also been noted [4].
Regarding the clinical grade of varicocele, a significant negative correlation has been established between ADC and varicocele grades, suggesting that ADC decreases as the grade of varicocele increases [1-3].
Preliminary data have reported that testicular FA was also sensitive for the diagnosis of clinical varicocele, with very good interobserver agreement. Specifically, FA (0.07) decreased in testes of infertile men with varicocele compared to healthy controls (0.11), with a cutoff of 0.08 providing high sensitivity (88%), specificity (93.5%), positive predictive value (91.6%), and negative predictive value (90.6%) for the diagnosis of clinical varicocele
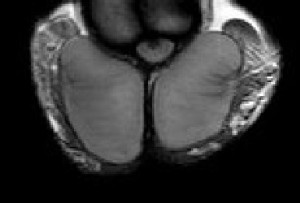
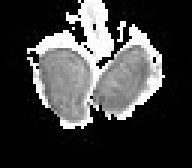

The mechanisms that act to decrease diffusion anisotropy in testes with varicocele are multifactorial. Testicular biopsies in these men have shown characteristic changes of depressed spermatogenesis, with a predominant decrease in the number of germ cells, resulting in an increase in the extracellular space, and thereby, in a decrease in the extent of directionality of water molecules diffusion. Coexisting hypoplasia and thinning of the germinal epithelium results in spermatic tubules enlargement and this further contributes to FA decrease, as luminal dilatation removes some directionality of diffusivity. Cellular debris, consisting of apoptotic germ cells might congest the spermatic tubules and therefore, impair directed diffusion. Another contributing factor is the increased deposition of interstitial fibrous tissue in testes of men with varicocele, that may destroy testes anisotropic structure [9-11].
Dynamic-Contrast Enhanced MRI
DCE-MRI, with semi-quantitative parameters has been explored for assessing testicular perfusion in infertile men with varicocele [6]. This study demonstrated significant differences in perfusion between infertile men with clinical varicocele and healthy controls. Specifically, testes in the varicocele group showed high values for maximum enhancement, maximum relative enhancement, and wash-in rate, detected as avid and fast, early contrast enhancement
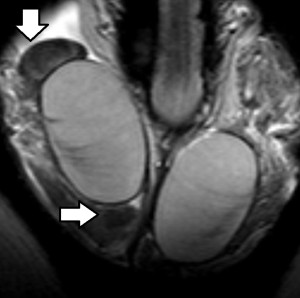
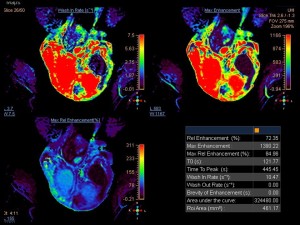
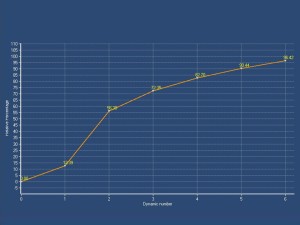
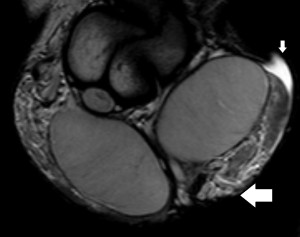
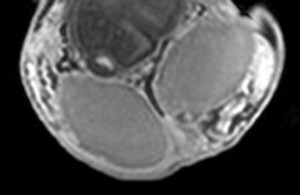
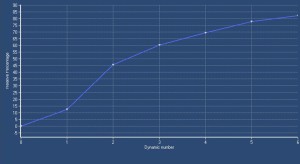
The rapid contrast uptake in varicocele-affected testes is likely due to factors such as arterial vasoconstriction, increased vascular permeability, and possibly elevated angiogenesis [12,13]. Arterial vasoconstriction due to interstitial testicular fibrosis and thickening of the interstitial blood wall vessels and also developed to counteract to the increased arterial pressure caused by elevated venous pressure, correlates to the increase in intratesticular blood flow resistance seen in clinical varicocele, during the early phases of dynamic imaging.Testicular angiogenesis represents another possible explanation for the early, strong vascularization of testes in infertile men with varicocele. Studies have demonstrated that hypoxia-inducible factor 1-alpha (HIF-1α), and vascular endothelial growth factor (VEGF) are overexpressed in males with varicocele. Under hypoxic states, such as varicocele, HIF-1α/VEGF expressions activate the endothelial cells, resulting in increased angiogenesis [13].
Proton Magnetic Resonance Spectroscopy
Testicular 1H-MR spectra of infertile men with clinical varicocele have shown a decrease in concentrations of choline (Chol), myoinositol (ml), Glx complex, and lipids

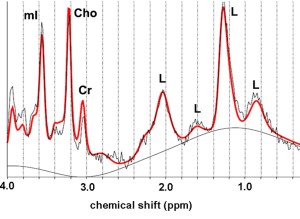
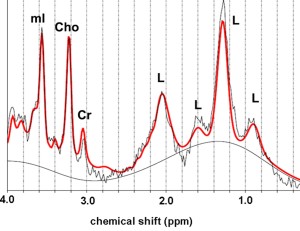
Choline represents an essential component of phospholipid membrane synthesis and a metabolic marker of cell membrane density and integrity, as well as of cellular proliferation. Testicular Cho levels closely correlate with the process of spermatogenesis and are mainly related to the number of proliferating germ cells. Decrease in the number of germ cells, due to impairment of spermatogenesis explains the lower concentrations of Cho in testes of infertile men with varicocele [14].
Myo-inositol is a cyclic sugar that is produced in the testes. The role of this metabolite in spermatogenesis is multifactorial, including the osmoregulation of the seminiferous tubules, the expression of proteins, that are essential for embryogenetic development and sperm chemotaxis and the maintenance of sperm motility, capacitation, and acrosome reaction. In addition, mI improves the mitochondrial function and prevents the apoptosis of the spermatozoa, due to its antioxidant behavior. The enzymes involved in mI synthesis are expressed in the meiotic germ cells and Sertoli cells and the mI transporter is located in the Sertoli cells. Arrest of spermatogenesis, with a reduction of germ cells and Sertoli cell dysfunction, with reduced number of Sertoli cells explain the low levels of mI in testes with varicocele [14,15].
Glutamate represents an essential amino acid. Although a high expression of Glu receptors inside the seminiferous tubules and in mature spermatozoa has been reported, the exact role of this metabolite in spermatogenesis is poorly understood. The decrease in testicular Glu in varicocele is probably related to changes in the metabolic pathways of glucometabolism and amino acid metabolism. In human testes, lactate (Lac) is the main energy source for germ cells, which is produced in the Sertoli cells, mostly through glucose metabolism. Glutamate represents an important precursor for the production of high amounts of Lac. Another possible explanation is related to the increased levels of OS and the decrease in concentrations of antioxidant agents, including a Glu-based system in testes with varicocele [14,16].
Lipids play a key role in spermatogenesis [17]. The development of mature spermatozoa requires highly specialized membrane function, using lipids as the main energy basis. Low concentrations of PUFAs have been reported in men with impaired spermatogenesis compared with normozoospermic population, and this was also observed in testes of infertile men with clinical varicocele. The above changes may be correlated to the high levels of OS in testes with varicocele, as human testes are susceptible to OS, due to their high energy and oxygen demand, and the abundance of PUFAs. Cholesterol represents one of the main lipids in testes and the principal precursor in testosterone synthesis, occurring in Leydig cells. Leydig cell dysfunction, with reduced number of Leydig cells in testes with varicocele results in low levels of cholesterol, and therefore, further contributes to the overall reduction in lipid concentrations [18].