PI-RADS
PI-RADS is the cornerstone of prostate MRI reporting. It provides a structured framework for detecting and localizing clinically significant prostate cancer (csPCa). The system uses a five-point scale to classify lesions based on their likelihood of being csPCa, ranging from PI-RADS 1 (highly unlikely) to PI-RADS 5 (highly likely) (3).
Key findings from studies have shown that PI-RADS significantly improves the detection of csPCa while reducing overdiagnosis of indolent tumors (2).
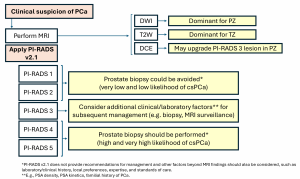
PI-RADS primarily targets treatment-naïve patients with suspected PCa and should be used before biopsy. It relies on T2-weighted imaging (T2W), Diffusion-Weighted Imaging (DWI), and Dynamic Contrast-Enhanced (DCE) sequences to evaluate lesions. The role of DCE is debated, with some guidelines suggesting biparametric MRI (bpMRI) as an alternative for biopsy-naïve patients. The score is zone-specific, meaning that assessments differ for lesions in the peripheral (PZ) and transition zone (TZ). DWI is the dominant sequence when assessing the PZ while T2W is dominant for the TZ. DCE plays a minor role and is used to upgrade (or not) an indeterminate PZ PI-RADS 3 lesion to PI-RADS 4.
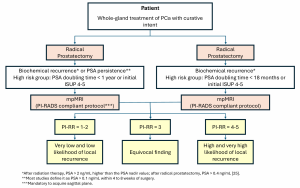
It is generally accepted that biopsy may be considered for patients with PI-RADS 4–5 and avoided for PI-RADS 1-2, while other clinical factors should be considered for biopsy decisions in patients with PI-RADS 3 lesions (Figure 2).
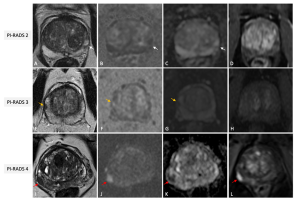
No more than four lesions should be described according to PI RADS v2.1, and all of them should be reported in a standardized graphical diagram (representing the base, mid gland, and apex in an axial plane), and explicitly measured (in mm), to standardize MRI review, inter-specialty communication, and targeting of lesions at biopsy.
However, it must be noted that PI-RADS v2.1 does not include possible biopsy options, as these must be based on a thorough risk assessment of the patient and strongly depend on local expertise and standard of care.
PI-QUAL v2
The accuracy of prostate MRI depends on image quality, which can be influenced by patient factors such as movement, rectal air, or the presence of implants (5). PI-QUAL was developed to standardize image quality assessment (6). (Figure 4)
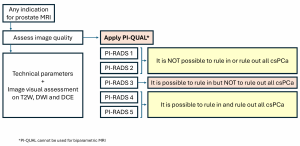
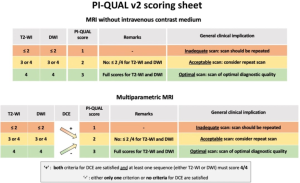
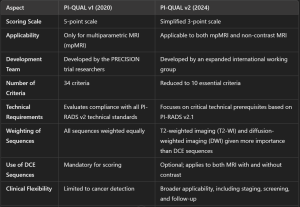
The PI-QUAL v2 introduces a simplified 3-point scale to evaluate both multiparametric MRI (mpMRI) and non-contrast MRI. The new system addresses limitations of the first version (PI-QUAL v1), such as excluding non-contrast MRI and using a complex 5-point scale (4).
PI-QUAL v2 evaluates image quality in three categories:
- T2-weighted imaging (T2-WI)
- Diffusion-weighted imaging (DWI)
- Dynamic contrast-enhancement (DCE)
MRI scans are assigned a score between 1 and 3:
- Score 1: Inadequate quality.
- Score 2: Acceptable quality.
- Score 3: Optimal quality.
PRECISE v2
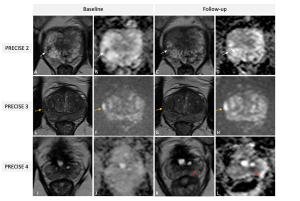
PRECISE is specifically designed for patients on active surveillance (AS). Eligibility for AS involves a biopsy Gleason score≤3+4, clinical stage≤ T2b and a serum PSA level <10ng/ml, but criteria vary across guidelines and practices. PRECISE system is not meant to be used for the follow-up of patients with negative biopsies, nor to evaluate treatment response. It monitors radiological changes in lesions over time, helping to predict disease progression (8). (Figure 6)
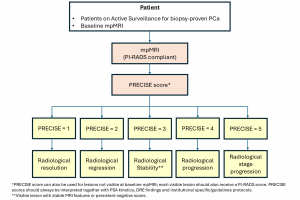
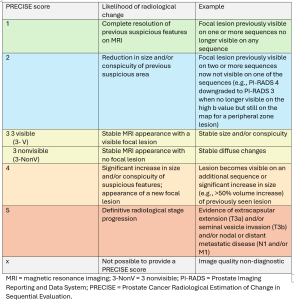
The system assigns a score from 1 to 5, with lower scores indicating stability or regression and higher scores suggesting progression.
PRECISE 3 (stable disease), according to PRECISE v2, is divided into two subcategories (7):
- 3-V (Visible): for stable, visible lesions on MRI.
- 3-NonV (Non-visible): for stable, non-visible disease.
A new score, PRECISE-X, has been introduced to indicate cases where image quality is inadequate, making it impossible to provide an accurate score.
The PRECISE 5 score is clarified as indicating progression to stage T3a or higher, signifying extracapsular extension or advanced disease stages, including nodal or distant metastases. (Figure 7 and table 2)
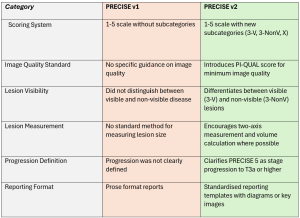
For each lesion, it is imperative to determine its appearance compared to the baseline scan, its current visibility status, and its size, which can be calculated according to different definitions (i.e., single diameter, biaxial diameter, ellipsoid formula or planimetry). For patients with visible target lesions measurements should be done on the dominant sequence as per PI-RADS version 2.1. (Figure 8)
PI-RR
For patients who have undergone radical prostatectomy or radiation therapy, the risk of biochemical recurrence (BCR) remains. PI-RR provides a standardized framework for detecting local recurrence through MRI (9). The system uses a five-point scale to classify the likelihood of recurrence based on anatomical and functional imaging findings (10). (Figure 9)
PI-RRscores of 1 and 2 are assigned when no abnormalities are detected or when “benign” findings are identified, such as fibrotic tissue or residual benign prostatic hyperplasia nodules. PI-RR scores 4 or 5 should be assigned according to primary tumor location. A score of 5 should be given when the lesion occurs at the primary tumor site; alternatively, a score of 4 applies if the finding appears in a different location or when the primary tumor location is unknown (11). (Figure 10)
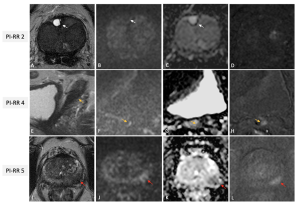
PI-RR has shown high diagnostic accuracy in detecting local recurrences, particularly when combined with DWI and DCE sequences. However, its clinical adoption is hindered by the increasing use of Prostate-Specific Membrane Antigen (PSMA) PET scans for BCR assessment.
The main disadvantage of the PI-RR system is that it assesses the risk of recurrence only within the prostate gland or prostate bed after treatment of the entire gland.
Recently, two scoring systems have been proposed to evaluate the likelihood of residual/recurrent disease after focal therapy (Prostate Imaging after Focal Ablation-PI-FAB- (12) and the Trans-Atlantic Recommendations for prostate Gland Evaluation with MRI after focal Therapy TARGET) (13) but both systems lack clinical validation.