Lymphomas arising in the thorax vary significantly by type; they are more common in Hodgkin's disease (ranging from 31% to 71%) than in non-Hodgkin's lymphoma (ranging from 13% to 23%) (1) , with HL demonstrating a predilection for young adults and NHL showing a broader age distribution. While HL classically originates in the lymph nodes, NHL often arises from extranodal sites, including the lungs, pleura, and mediastinal structures. The clinical presentation varies: HL may manifest with constitutional “B symptoms” (e.g., fever, night sweats), whereas NHL can present with nonspecific respiratory symptoms such as cough or dyspnea, complicating early diagnosis.
Imaging serves as the cornerstone of evaluation, bridging the gap between clinical suspicion and histopathological confirmation. Accurate staging, guided by the Lugano classification system, hinges on radiological findings to determine disease extent, prognosis, and therapeutic strategies, including chemotherapy, radiation, or immunotherapy.
A-Radiological Modalities: Strengths and Applications
- Computed Tomography (CT)
CT remains the primary modality for initial assessment due to its superior spatial resolution and ability to delineate anatomical details. Contrast-enhanced CT excels in identifying lymphadenopathy, parenchymal lesions, and complications such as airway compression or superior vena cava syndrome. In HL, contiguous mediastinal lymph node enlargement is characteristic, often involving the anterior and middle compartments. NHL, conversely, may present with non-contiguous nodal disease or extranodal masses, such as solitary pulmonary nodules or diffuse infiltrates mimicking pneumonia.
Limitations include inadequate differentiation between active lymphoma and post-treatment fibrosis, as well as difficulty distinguishing lymphoma from metastatic carcinoma or granulomatous diseases.
- Positron Emission Tomography (PET)/CT
Integrated PET/CT combines metabolic and anatomical data, revolutionizing lymphoma management. Fluorodeoxyglucose (FDG) avidity, quantified via standardized uptake values (SUVs), reflects tumor aggressiveness and guides biopsy targeting. In HL, PET/CT detects subcentimeter nodes missed by CT alone, while in NHL, it identifies extranodal sites such as pericardial or chest wall involvement (2).
The Deauville 5-point scale is pivotal for post-treatment response assessment, distinguishing residual active disease from inflammation. However, false positives may arise from infectious or inflammatory processes, and indolent NHL subtypes (e.g., marginal zone lymphoma) often exhibit low FDG avidity, reducing sensitivity.
- Magnetic Resonance Imaging (MRI)
MRI’s superior soft-tissue contrast makes it invaluable for evaluating chest wall invasion, spinal involvement, or pericardial disease. Diffusion-weighted imaging (DWI) and dynamic contrast-enhanced sequences provide functional insights, aiding in the detection of early treatment response. Nevertheless, motion artifacts from respiration and cardiac pulsation limit its utility in lung imaging.
- Ultrasound
Ultrasound plays a niche role in assessing pleural effusions and guiding thoracentesis. Complex septated effusions, often exudative, may suggest lymphoma involvement, though cytological confirmation remains necessary.
B-Hodgkin vs. Non-Hodgkin Lymphoma: Distinct Imaging Phenotypes
1.Hodgkin Lymphoma (HL): Radiologic Hallmarks
a) Lymph Node Involvement: A Topographic Signature
HL is characterized by contiguous, predictable spread along lymphatic chains. Imaging typically reveals enlargement of anterior mediastinal and paratracheal lymph nodes, a near-pathognomonic feature [Figure 1]. At diagnosis, 67% of patients exhibit intrathoracic lymphadenopathy, with a predilection for the superior mediastinum (99% of cases). Isolated hilar involvement is rare, and nodal calcifications—exceptional prior to treatment—may emerge post-radiotherapy (3).
The Ann Arbor-Cotswolds staging system guides prognosis: localized stages (IA-IIA) may be managed with radiotherapy alone, while advanced disease requires combination chemotherapy.
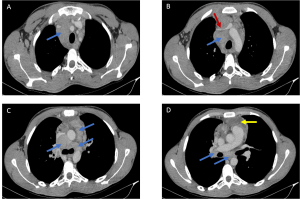
b) Pulmonary Parenchymal Involvement: Three Dominant Patterns
. Multiple Nodules: Present in 70% of cases, these ill-defined opacities, often associated with hilar lymphadenopathy, reflect direct tumor infiltration or lymphatic dissemination [Figure 2].
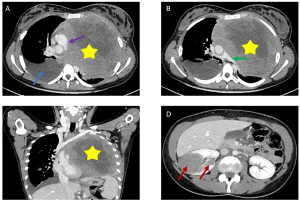
. Septal Reticulations: Secondary to lymphatic or venous obstruction by enlarged nodes, these interlobular lines may mimic pulmonary edema (4).
. Alveolar Consolidations [Figure 3]: Air bronchograms within consolidated areas can resemble bacterial pneumonia, necessitating biopsy for confirmation (4).
c) Thoracic Complications
Pleural effusions, resulting from lymphatic obstruction rather than direct pleural invasion, are common. Bulky mediastinal disease may cause superior vena cava syndrome, tracheal compression, or chest wall masses (e.g., within pectoral muscles) (5).
2.Non-Hodgkin Lymphoma (NHL): Radiologic Heterogeneity
a) Histologic Diversity and Imaging Implications
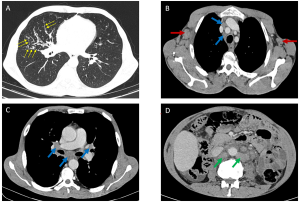
Unlike HL, NHL comprises a broad spectrum of subtypes (low- to high-grade), each dictating distinct presentations. Only 43% of patients present with thoracic involvement at diagnosis, with mediastinal lymphadenopathy in fewer than 50% of cases. Aggressive NHLs (e.g., diffuse large B-cell lymphoma) manifest as bulky mediastinal masses (>10 cm) causing compression [Figure 4], while indolent forms (e.g., follicular lymphoma) favor isolated lymphadenopathy.
Large lymph node masses may exhibit heterogeneity with complex low attenuation, indicative of necrosis, hemorrhage, or cystic degeneration (2).
b) Parenchymal Involvement: A Polymorphic Spectrum
Though rare (<5% of cases), pulmonary infiltration may occur without associated lymphadenopathy, particularly in primary pulmonary lymphomas. Patterns include:
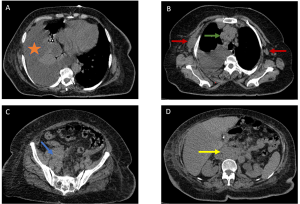
- Cavitary Nodules [Figure 5]: Characteristic of angiocentric lymphoma (formerly lymphomatoid granulomatosis), these lesions occur in 25% of cases (6).
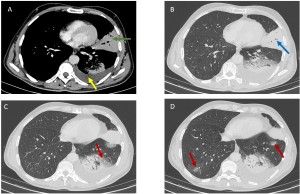
- Alveolar Masses: Seen in mucosa-associated lymphoid tissue (MALT) lymphomas, these slow-growing lesions carry a favorable prognosis (5-year survival >80%) (7).
- Reticulonodular Infiltrates: Associated with peribronchovascular thickening, these often result from direct extension from hilar nodes.
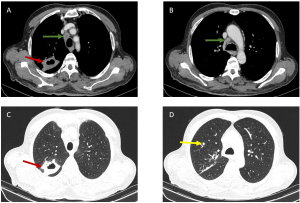
c) Complications and Rare Involvement
Pleural effusions typically coexist with mediastinal lymphadenopathy [Figure 6]. Thoracic wall invasion, more common in recurrent disease, presents as infiltrative masses with osseous or muscular destruction.
C- Clinical Implications and Therapeutic Considerations
- Staging and Prognostication
Imaging determines disease stage, which directly impacts therapy. For example, localized HL (stage I–II) may be treated with abbreviated chemotherapy and involved-site radiation, while advanced disease requires multi-agent regimens. In NHL, PET/CT findings influence the International Prognostic Index (IPI), guiding risk-adapted therapies.
- Treatment Response Assessment
Interim PET/CT after 2–4 chemotherapy cycles predicts long-term outcomes. A Deauville score of 4–5 necessitates treatment escalation, whereas scores of 1–3 may permit de-escalation to reduce toxicity. Post-treatment surveillance imaging, however, remains controversial due to radiation exposure and cost-effectiveness concerns.
- Complications and Follow-Up
Imaging detects therapy-related complications, such as radiation pneumonitis or chemotherapy-induced cardiomyopathy. Late relapses, particularly in NHL, require long-term monitoring via low-dose CT.