EMBRYOLOGY
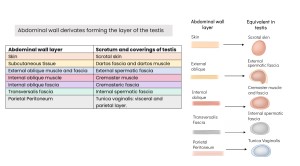
The testes originate from the gonadal ridge, derived from intermediate mesoderm. Initially located in the posterior abdominal wall, they descend through the inguinal canal into the scrotum guided by the gubernaculum testis, carrying layers of the abdominal wall that form the testicular coverings.
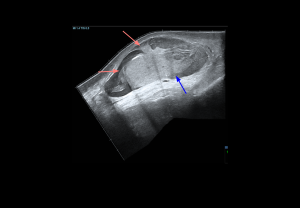
ULTRASOUND ANATOMY OF THE TESTES
The testes, suspended in the scrotum, are ovoid and obliquely oriented, with the left usually slightly lower. On ultrasound, adult testes measure 3–5 cm in length, 2–4 cm in width, ~3 cm anteroposteriorly, with an average volume of 15–20 mL.
- Grey-scale: adult testes show homogeneous, medium-level echotexture with smooth, well-defined margins.
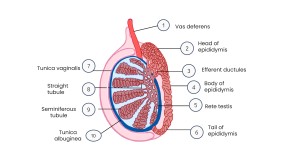
Testicular coverings
Tunica albuginea:
Dense fibrous capsule that envelops the testicular parenchyma, providing structural support and protection.
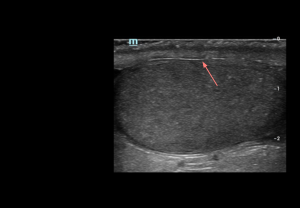
- Grey-scale: appears as a thin, continuous echogenic line demarcating the testis. Intact tunica albuginea is a key imaging feature in the assessment of scrotal trauma.
Tunica vaginalis
Serous membrane derived from the processus vaginalis that envelops the tunica albuginea, forming a closed peritoneal sac with visceral and parietal layers.
- Grey-scale: appears as a fine echogenic line; the layers are usually indistinguishable unless fluid, as in hydrocele, separates them.
Intratesticular parenchyma
Seminiferous tubules:
Convoluted tubules continuing as straight tubules (tubuli recti) that converge toward the mediastinum testis and drain into the rete testis. Functional units of spermatogenesis.
Collecting duct system
Rete testis:
Network of channels connecting the tubuli recti to efferent ductules; appears on grey-scale ultrasound as small, clustered anechoic or hypoechoic tubular or cystic spaces without mass effect.
Efferent ductules: approximately 10–20 ductules originate from the rete testis and project toward the epididymal head, opening into the epididymal duct to form the ductus deferens
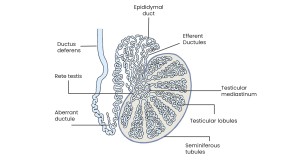
Mediastinum testis
Thickened region at the posterosuperior aspect of the testis.
- Grey-scale: appears as a linear or band-like hyperechoic structure along the longitudinal axis of the testis.
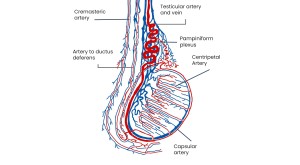
Vascularization of the testes
Arterial supply:
Mainly from the testicular arteries (abdominal aorta), with contributions from the cremasteric artery and artery of the ductus deferens. Capsular arteries supply the tunica albuginea and branch centripetally and centrifugally to perfuse the parenchyma and seminiferous tubules.
Venous drainage:
Testicular veins form the pampiniform plexus; the right drains into the inferior vena cava, the left into the left renal vein. Scrotal veins drain via the inferior epigastric and deep pudendal veins at the deep inguinal ring.
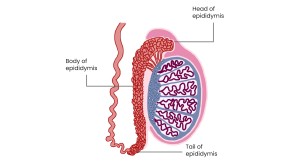
ULTRASOUND ANATOMY OF THE EPIDIDYMIS
Is a 6–7 cm tubular structure along the superolateral border of the testis, divided into three parts:
- Head: converging efferent ductules at the superior pole, extending posteriorly; heterogeneous, isoechoic/slightly hyperechoic to testicular parenchyma; 5–12 mm diameter.
- Body: along the posterior testicular margin; isoechoic to testicular parenchyma; 2–4 mm diameter.
- Tail: at the inferior pole, continues as the ductus deferens; echogenicity similar to testis; 2–5 mm diameter.
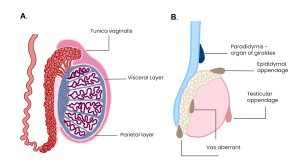
TESTICULAR AND EPIDIDYMAL APPENDAGES
Vestigial derivatives of the mesonephric (Wolffian) system, with no functional significance.
- Appendix of the epididymis: remnant at the epididymal head; typically isoechoic on grey-scale.
- Appendix of the testis (hydatid of Morgagni): ovoid structure at epididymal head/superior pole; isoechoic to slightly hypoechoic on ultrasound.
- Paradidymis: superior to the epididymal head.
- Aberrant inferior duct: blind-ending diverticulum from the epididymal tail
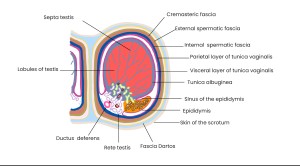
ANATOMY OF THE SCROTAL SAC
The scrotum is a cutaneous sac enclosing a testis, its epididymis, and the distal segment of the spermatic cord. Its layers include:
- Skin: Thin, extensible, with transverse folds and a median raphe.
- Dartos tunic: Smooth muscle forming the suspensory apparatus and scrotal septum; contributes to contraction, contour, and thermoregulation.
- External spermatic fascia: from the external oblique aponeurosis.
- Cremasteric fascia and muscle: from the internal oblique muscle; envelops the spermatic cord.
- Internal spermatic fascia: Continuation of the transversalis fascia; forms the scrotal ligament (gubernaculum remnant).
- Tunica vaginalis
ANATOMY OF THE SPERMATIC CORD
Extends from the testis to the deep inguinal ring, enclosed by fascial layers from the anterior abdominal wall. It contains the ductus deferens, testicular and cremasteric arteries, pampiniform plexus, nerves, and lymphatics.
- Grey-scale: appears as a homogeneous tubular structure with parallel serpiginous channels representing the vessels and ductus deferens.
ULTRASOUND ASSESSMENT OF THE ACUTE SCROTUM
Comprehensive evaluation includes grey-scale, colour, power, and spectral Doppler of the testes, epididymides, and scrotal contents.
Technique:
- Patient positioning: Supine, hips and knees flexed in external rotation; scrotum elevated on a folded towel; penis draped against the lower abdomen.
- Equipment: High-frequency linear transducer (7–14 MHz).
- Scanning planes: Longitudinal and transverse images of each testis; comparative transverse view at the midline for simultaneous evaluation.
- Parameters: Morphology, size, echogenicity, and vascular flow.
Initial assessment: Examine scrotal skin for symmetry, thickening, or edema. Start with the asymptomatic testis to optimize Doppler and compare findings with the contralateral side.
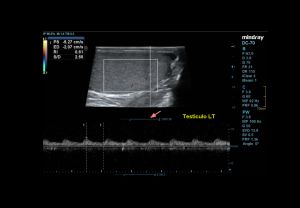
DOPPLER EVALUATION
Assessment of testicular perfusion is a critical component in acute scrotal pain.
Technical optimization:
- Use the lowest velocity scale and low wall filter to detect slow flow.
- Maximise colour gain for sensitivity, avoiding excessive noise.
Flow characteristics:
- Intratesticular arteries: Low-resistance waveform with continuous diastolic flow; peak velocities 3–5 cm/s; RI 0.4–0.7.
- Cremasteric and deferential arteries: High-resistance patterns.
- Veins: Non-pulsatile, low-velocity continuous flow.
- Centripetal and transmediastinal arteries: Low-resistance patterns.
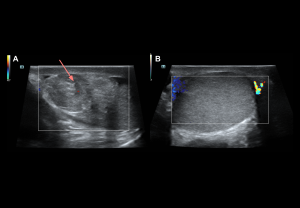
ACUTE SCROTUM
It is a urological emergency marked by sudden scrotal pain, swelling, and erythema, requiring prompt evaluation to prevent testicular loss. Etiologies include vascular, inflammatory, and traumatic disorders.
Vascular Disorders
Testicular torsion:
Twisting of the testis around the spermatic cord, leading to vascular compromise.
Types:
- Supravaginal or extravaginal (<5%): Common in neonates
- Intravaginal (≈95%): Associated with the “bell-clapper deformity
Time-sensitive management: testicular salvage is 100% if detorsion occurs within 6 hours, ~75% at 12 hours, and <50% between 12–24 hours.
Clinical features: Sudden pain radiating to the ipsilateral fossae, swelling, absent cremasteric reflex, negative Prehn’s sign, and horizontal orientation.
Grey-scale:
- Early (<6 h): normal echogenicity
- Acute (<6 h): testis enlargement, thickened scrotal coverings, reactive hydrocele, altered epididymal echogenicity or position.
- Late: reduced size, heterogeneous echotexture; hypoechoic areas
Doppler findings:
- Degree of torsion:
- Complete (≥360°): Usually absent intratesticular flow.
- Partial (<360°): Residual intratesticular flow may be detectable.
- Spectral Doppler: Increased resistive index (RI) and possible diastolic flow reversal, whirlpool sign of spermatic cord
- Late (>24 h): Periscrotal hyperaemia reflects inflammation.
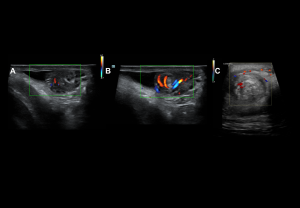
Appendiceal Torsion
- Clinical features: focal upper-pole scrotal pain, firm non-mobile testis, “blue dot sign.”
- Grey-scale: well-defined ovoid extratesticular mass, hyperechoic with central hypoechoic area, reactive hydrocele, normal testis echotexture
- Doppler findings: avascular appendage, preserved testicular perfusion, perilesional hyperaemia.
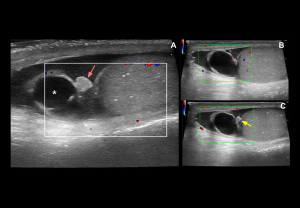
Testicular infarction
Grey-scale:
- Acute phase: mild enlargement with preserved echogenicity.
- Advanced phase:
- Ischaemic infarction: Focal, well-defined hypoechoic areas, with homogeneous echotexture.
- Haemorrhagic infarction: Heterogeneous echotexture with hyperechoic foci.
Doppler findings:
- Absent/reduced intratesticular arterial flow, diastolic flow reversal
- Elevated resistive index (RI > 0.7).
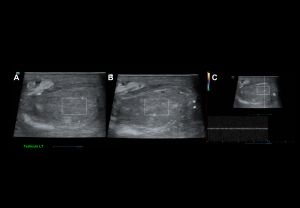
Inflammatory Disorders
Epididymo-orchitis
The most common cause of acute testicular pain in adults.
- Clinical findings: tender enlarged testis, scrotal wall thickening, erythema, fever, positive Prehn’s sign, preserved cremasteric reflex.
- Grey-scale: Enlarged heterogeneous epididymis, testicular enlargement with heterogeneous hypoechogenicity, reactive hydrocele.
- Doppler: Diffuse hyperaemia, low-resistance arterial flow (RI: epididymis <0.7, testis <0.5).
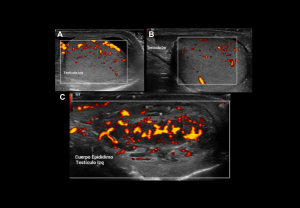
Intratesticular Abscess
Complication of untreated epididymo-orchitis.
- Grey-scale: Round/ovoid intratesticular or intraepididymal lesions, poorly defined, heterogeneous.
- Doppler: Avascular lesion with peripheral hyperaemia
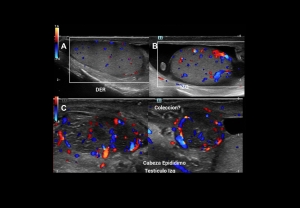
Pyocele:
Infected hydrocele post-inflammation or trauma.
- Grey-scale: Loculated fluid collection with echogenic debris, reactive hydrocele and scrotal wall thickening
- Doppler: Peripheral hyperaemia, absent internal flow.
Fournier`s Gangrene
Necrotising fasciitis of genitalia and perineum, often in diabetics (~50%). Ultrasound is valuable for early evaluation of testicular viability.
- Grey-scale: Thickened, oedematous scrotal layers; echogenic foci and acoustic shadowing from gas.
- Doppler: Increased scrotal wall vascularity; preserved intratesticular flow.
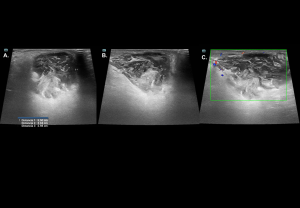
Traumatic Disorders
Testicular trauma
Extratesticular lesions
- Hydrocele: anechoic fluid between tunica vaginalis layers; no Doppler flow.
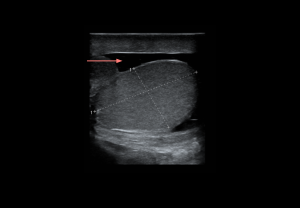
- Hematocele: heterogeneous fluid collection with echogenicity varying by chronicity; no internal flow. Large collections may reduce testicular perfusion by compression.
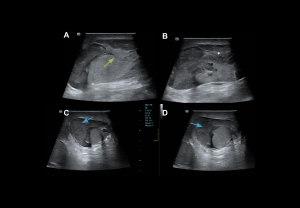
Intra-/extratesticular lesions
- Hematoma: well-defined avascular lesion; echogenic in the acute phase, hypoechoic in the chronic phase.
Intratesticular lesions
Integrity of the tunica albuginea determines management:
- Testicular fracture: Intact tunica albuginea, linear hypoechoic band, preserved peripheral flow.
- Testicular rupture: Disrupted tunica albuginea with parenchymal extrusion, heterogeneous echotexture, absent/reduced flow.