
1.Pathogenesis of adenomyosis [6] (Figure 1)
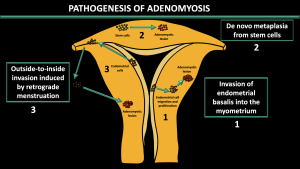
Invasion of endometrial basalis into the myometrium
-Invasion of altered endometrial basalis cells into myometrium through an injured or abnormal junctional zone.
-Associated with junctional zone thickening and internal adenomyosis
De novo metaplasia from stem cells
-Forming of ectopic endometrium by de novo metaplasia of embryonic epithelial progenitors (remnants) or by differentiation of adult endometrial stem cells that migrate to the myometrium.
Outside-to-inside invasion induced by retrograde menstruation
-Implantation of stem cells through retrograde menstruation and invasion of the outer myometrium
-Associated with external adenomyosis
2.Junctional Zone [5] (Figure 2)
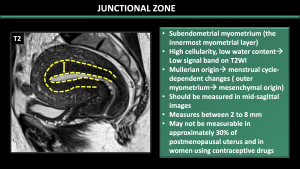
The JZ corresponds to the innermost layer in the myometrium. Due to high cellularity, larger nuclear area, looser extracellular matrix, and lower water content, JZ appears hypointense on T2W images. In comparison to the outer myometrium, this layer is müllerian in origin and affected by cyclic-dependent alterations. Hence, one of the main contributors to the changes in the thickness of the junctional zone, as observed on MRI, is the hormonal variation in the female reproductive cycle [5].
Pitfalls:
*The pseudo-thickening of the JZ during the menstrual phase can lead to a misdiagnosis of adenomyosis, so it is recommended to avoid scanning during this time.
*Hormonal conditions such as Pregnancy and pre-menarcheal age can hinder the identification of the JZ.
*JZ may not be measurable in approximately 30% of postmenopausal uterus and in women using contraceptive drugs.
3.MRI criteria for adenomyosis [3,7,8]
Internal Adenomyosis (Figure 3,4)
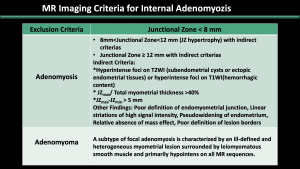
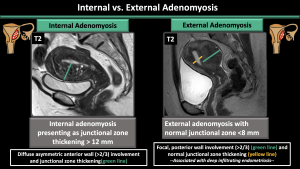
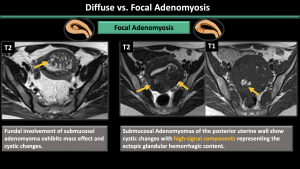
Internal adenomyosis is typically characterized by a low-signal-intensity area on T2-weighted images during MR imaging, resulting in the perception of a wider junctional zone. The areas displaying low signal intensity have been proven to correspond with the smooth muscle hyperplasia that accompanies the heterotopic endometrial tissue [7]. Junctional zone thickness > 12 mm, junctional zone thickness to myometrial thickness ratio > 40%, and the presence of intramyometrial cysts or hyperintense foci on T1WI (hemorrhagic components) are the most reliable imaging features of internal adenomyosis [7,8].
External Adenomyosis (Figure4)

External adenomyosis originates in the outer myometrium or may infiltrate from external sources, causing disruption of the serosa while sparing the JZ. In cases of external adenomyosis, the thickness of the junctional zone remains normal. External adenomyosis is characterized by a well-defined subserosal myometrial mass with low signal intensity, often accompanied by deep infiltrating endometriosis [3].
4.Classification and reporting proposal [3] (Figure5)
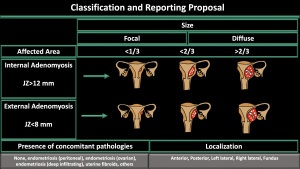
-Affected Area (internal or external) (Figure 4)
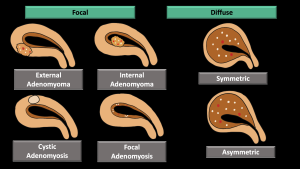
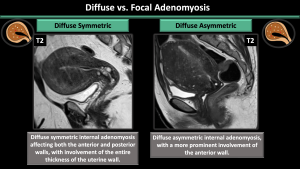
-Pattern (diffuse symmetric or asymmetric-focal) (Figure 6,7,8)
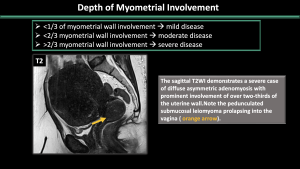
-Depth of myometrial involvement (<1/3 or <2/3 or >2/3) (Figure 9)
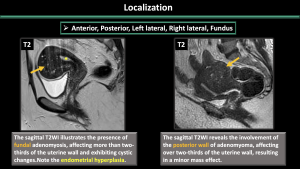
-Localization (anterior, posterior, left lateral, right lateral, fundal) (Figure 10)
-Concomitant pathologies (none, peritoneal endometriosis, ovarian endometrioma, deep infiltrating endometriosis, uterine fibroids, others) (Figure 11-17)
5.Concomitant Pathologies [1,2,3] (Figure 11-17)
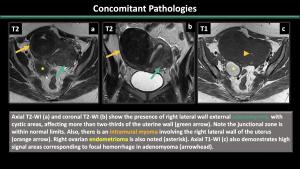
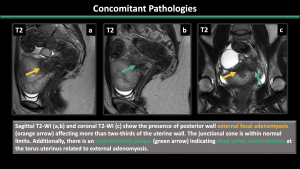
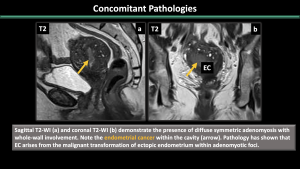
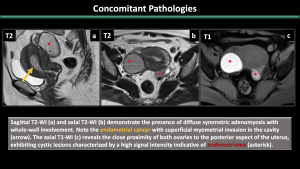
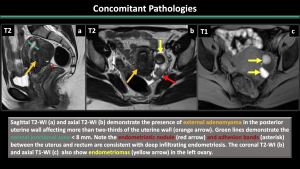
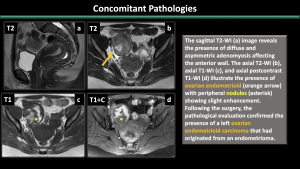
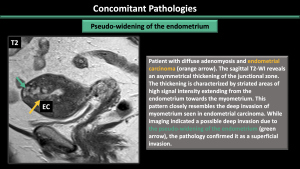
Estrogen-dependent pathologies, including endometriosis, leiomyomas, endometrial hyperplasia, endometrial cancer, and polyps, are commonly observed along with adenomyosis.
6.Atypical Presentation of Adenomyosis [1,2,3]
Adenomyoma and adenomyotic polyp (Figure 18,19)
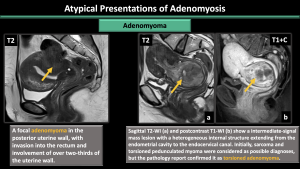
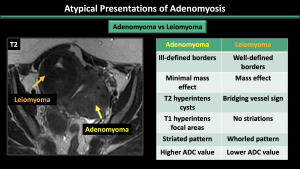
An adenomyoma is a concentrated grouping of adenomyotic glands, resembling a mass-like manifestation of adenomyosis. It can also grow as a polypoid mass within the endometrium, forming a polypoid adenomyoma.
*Ddx: Leiomyomas
Cystic adenomyosis (Figure 20-22)
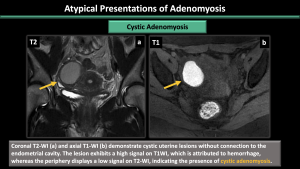
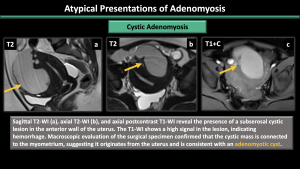

The cavity filled with hemorrhagic fluid, which is lined by endometrium and surrounded by myometrium, does not communicate with the uterine cavity.
*Ddx: accessory cavitated uterine mass (ACUM), endometrioma, rudimentary or cavitated uterine horns, adenomyosis with degenerated areas, degenerated leiomyomas
7.Mimickers of Adenomyosis and Potential Pitfalls [9]
Several benign conditions and malignant tumors have the potential to imitate adenomyosis, such as physiologic myometrial contraction, myometrial involvement by pelvic endometriosis, low-grade endometrial stromal sarcoma (LG-ESS), and lymphoma.
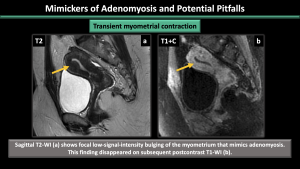
- Transient myometrial contraction: Physiological transient myometrial contraction can mimic adenomyosis. This appearance may no longer be present in subsequent images, while focal adenomyosis remains visible in subsequent images. (Figure 23)
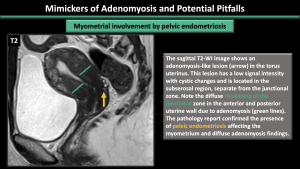
- Myometrial involvement by pelvic endometriosis: In the subserosal region, there may be lesions that resemble adenomyosis, distinct from the junctional zone. These lesions could be a result of pelvic endometriosis affecting the myometrium. (Figure 24)
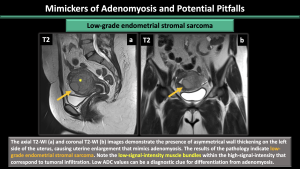
- Low-grade endometrial stromal sarcoma: A rare malignant mesenchymal tumor is causing uterine enlargement, with an ill-demarcated infiltrating myometrial mass. The myometrium infiltration is visually represented on T2W images by a heterogeneous intermediate signal with internal low-signal bands. (Figure 25)
- Lymphoma: Diffuse enlargement of the uterus is a result of secondary involvement. Low ADC values can be useful in distinguishing from adenomyosis.