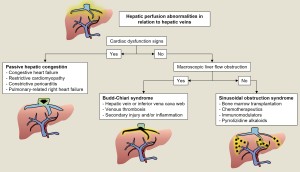
1. RELATED TO HEPATIC VEINS
Congestive hepatopathy
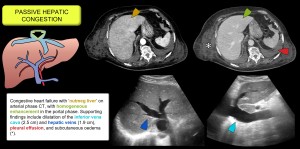
Occurs when increased venous pressure, usually due to right heart failure, cardiomyopathy or pericardial/valvular disease, is transmitted to the liver causing sinusoidal dilatation, hepatocyte atrophy and hepatomegaly with ascites and elevated bilirubin and liver enzymes.
Imaging showed hepatomegaly, dilatation of the hepatic and inferior caval veins, and loss of the triphasic Doppler pattern with increased phasicity in the portal vein. CT shows early enhancement of the cava and dilated hepatic veins due to contrast reflux from the right atrium and a heterogeneous liver pattern ('nutmeg liver'). Other findings include cardiomegaly and pericardial or pleural effusion. It may lead to chronic liver disease of cardiac origin [1,2]. Figure 1.
Fontan-associated liver disease
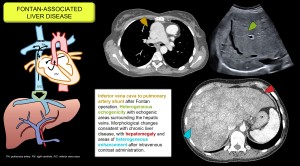
In this entity, hepatic congestion results from increased central venous pressure due to the direct connection between the systemic veins and the pulmonary arteries. Findings include heterogeneous parenchymal texture, echogenic nodules, caudate lobe hypertrophy, reticular enhancement pattern on CT and an increased incidence of HNF-like lesions and hepatocarcinoma [3]. Figure 2.
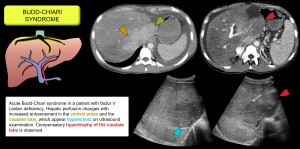
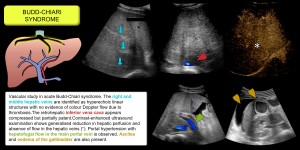
Budd-Chiari syndrome
Obstruction of the hepatic venous outflow due to thrombosis or less common causes (congenital or tumour vascular network) causes a heterogeneous pattern of enhancement due to sinusoidal dilatation secondary to increased venous pressure. This leads to ischaemia, cellular necrosis, hepatic dysfunction, portal hypertension and ascites.
- In the early stages, hepatic vein-dependent peripheral hypoattenuation is observed, while the caudate lobe (with independent venous drainage) and central areas near the inferior cava show early enhancement. Other findings include dilatation of the caudate lobe veins, splenomegaly, hepatoprophobic portal flow due to portal hypertension, increased hepatic artery calibre and vesicular oedema. Figures 3/4.
- In the chronic phase, peripheral atrophy, compensatory hypertrophy of the caudate lobe, intrahepatic collaterals, irregularity of the suprahepatic veins and nodular HNF-like lesions [4–6].
2. RELATED TO SINUSOIDS
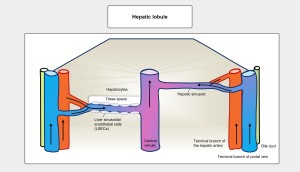
The hepatic artery and portal vein capillarise to form the hepatic sinusoids, which drain into the central vein, a tributary of the inferior vena cava. The functional unit of the liver is the hepatic lobule, which has a hexagonal structure with portal spaces at the peripheral corners. Blood flow is centripetal, from the periphery to the centre, through sinusoids lined by fenestrated endothelial cells that facilitate nutrient exchange with the hepatocytes. Between the sinusoids and the adjacent hepatocytes is the perisinusoidal space of Disse. Figure 5.
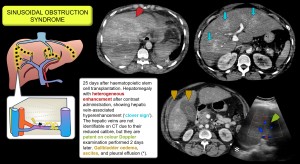
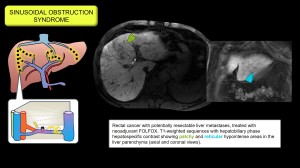
Sinusoidal obstruction syndrome (hepatic veno-occlusive disease)
It is caused by endothelial injury that creates gaps in the sinusoidal barrier, allowing cellular debris to enter the Disse space, dislodging endothelial cells, obstructing the sinusoids and leading to congestion, portal hypertension and liver dysfunction.
Common causes include myeloablative conditioning regimens prior to haematopoietic stem cell transplantation, chemotherapy with oxaliplatin (used in colorectal and pancreatic cancer), radiotherapy, and ingestion of herbal products containing pyrrolizidine alkaloids.
Previously known as veno-occlusive disease, central venous involvement is not essential but indicates greater severity [7,8].
Imaging findings: hepatomegaly, patchy CT enhancement with increased enhancement around the hepatic veins (clover sign), oedema of the gallbladder wall, invisible or narrow hepatic veins (<3 mm), portal dilatation >12 mm (reduced flow or hepatopetal) and hepatic artery dilatation. Progressive splenomegaly may also be seen. On gadoxetic acid MRI, hypointensity in the hepatobiliary phase reflects hepatocyte damage with a reticular pattern and peripheral predominance [9–13]. Figures 6/7.
A summary of the entities mentioned so far is summarised in Figure 8.
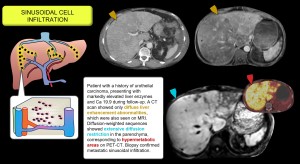
Sinusoidal cell infiltration
It includes histiocytic proliferation, neoplastic infiltrates and mastocytosis, with nodular, massive, portal or sinusoidal patterns depending on the disease. In sinusoidal infiltration of metastatic carcinoma, the liver fills with tumour cells without forming classic masses, causing liver failure without obvious clinical metastases. MRI diffusion sequences are key to its detection [14–16]. Figure 9.
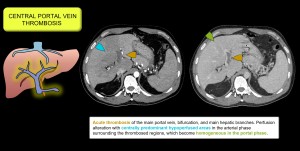
3. RELATED TO THE CENTRAL PORTAL VEIN
Thrombosis of the main portal vein and its branches produces a perfusion abnormality consisting of a large area of decreased enhancement in the perihilar region and relative enhancement in the peripheral areas, which may normalise in the late phase [17]. Figure 10.
4. RELATED TO THE HEPATIC ARTERY
The liver has a dual blood supply: 75% from the portal vein and 25% from the hepatic artery. Impaired portal flow triggers compensatory arterial hyperperfusion, leading to transient hepatic attenuation differences (THAD) in the early post-contrast phase. The main causes of THAD are:
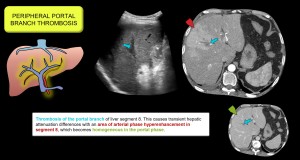
- Peripheral portal vein thrombosis. Causes perfusion abnormalities due to compensatory arterioportal shunts. Figure 11.
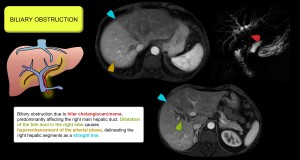
- Intrahepatic biliary dilatation. Obstructs the peribiliary plexus, reducing portal flow and compensating with increased arterial flow. Figure 12.
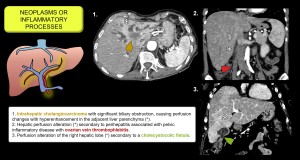
- Neoplasms or inflammatory processes (cholangitis, cholecystitis, etc.). Increase sinusoidal pressure, reduce local portal flow and produce arterioportal shunts with regional arterial hyperaemia. Figure 13.
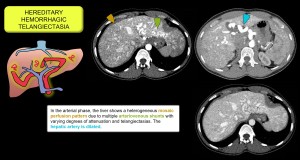
- Intrahepatic vascular shunts. Arterioportal communications that increase regional arterial flow and pressure gradient, producing THAD. They are multiple in Rendu-Osler-Weber disease [17,18]. Figure 14.
5. RELATED TO A THIRD INFLOW
Refers to vessels other than the portal vein and hepatic artery that provide additional flow to the liver. This flow may come from anomalous gastric veins, epigastric, paraumbilical (Sappey's and Burow's veins) or cystic veins.
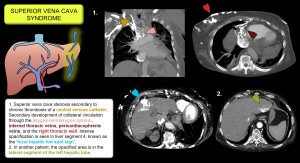
- Systemic venous obstruction may cause hepatic pseudolesions with arterial hyperenhancement. The hepatic hot spot sign is a vascular pseudolesion suggestive of superior vena cava obstruction. On contrast-enhanced CT, it appears as a focal hyperdense area in segment 4 of the liver, caused by shunting of blood and contrast through internal mammary and epigastric veins communicating with paraumbilical veins, which increases focal flow in the left hepatic lobe. Figure 15.
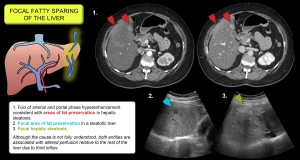
- It is thought to be responsible for focal fat infiltration and preservation [17,19,20]. Figure 16.