This prospective observational study was approved by the institutional ethics committee of our hospital.
Study Patients
This was an interim analysis of 29 patients, out of which 31% were females and 69 % were males. These patients , referred to the radiology department for CTPA with a clinical suspicion of pulmonary thromboembolism, underwent DDR in the supine position prior to the CTPA. A transthoracic 2D echocardiography (TTE) was done as standard of care as part of the workup of PTE to evaluate cardiac function. Patients referred for CTPA for non PTE indications were excluded from the study. CTPA were evaluated for pulmonary thromboembolism by experienced radiologists having more than 15 years of experience.
Dynamic Digital Radiography
All patients underwent a chest imaging on the DDR with a 7–10 second inspiratory breath-hold using a flat-panel detector (Konica Minolta AeroDR HD2) and pulsed x-ray generator (Shimadzu RADspeed Pro). Imaging was performed supine, with anteroposterior x-ray pulses (80–90 kV tube voltage; 80-100 mA tube current; 15 frames per second; 6.3 msec pulsed x-ray duration; 0.3 mm Cu additional filter; 1.5 m source-to-image distance; 1062 × 1062 pixel matrix; 400 × 400 μm pixel size; 42.5 × 42.5 cm whole image area).
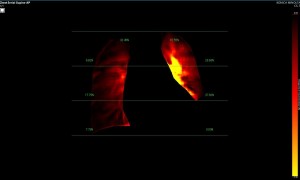
Dynamic pulmonary perfusion maps were generated from sequential DDR images using a prototype intelligent workstation (Konica Minolta) and proprietary software. The software calculated the temporal change in pixel value (x-ray translucency) for each pixel. The end-diastolic phase was automatically identified from pixel value changes in the heart. These temporal pixel value changes from end-diastole were color-coded and displayed as dynamic perfusion images. Small (dark on radiography, high x-ray translucency) and large (bright on radiography, low x-ray translucency) pixel values, representing low and high blood volume respectively, were interpreted as reflecting pulmonary circulatory changes. Maximum Intensity Projection (MIP) images, derived from dynamic perfusion images spanning one cardiac cycle, served as lung perfusion maps. Lung regions were automatically contoured using an edge detection algorithm and manually adjusted as needed.

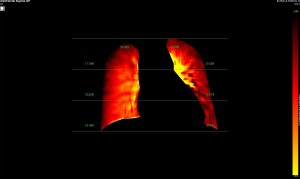
Image Analysis
DDR images and CTPA were analysed by experienced radiologists. DDR assessment was based on dynamic lung perfusion images. Conventional chest radiographs provided lung border reference along with evaluation of lung abnormalities (mass/nodule, any other opacity, pleural effusion etc). A sharply defined, wedge-shaped abnormality on the dynamic perfusion image or lung perfusion map, coupled with normal lung findings on the chest radiograph, constituted an embolic perfusion defect. The presence of at least one such defect resulted in a final diagnosis of PTE.
Study Limitations
One of the major limiting factors was a poor breath hold which compromised the image quality and interpretation of the images as it hampered the ability of the software to analyse pulmonary perfusion. Lung motion caused improper assessment of x ray attenuation and therefore created an improper pixelation for analysis.
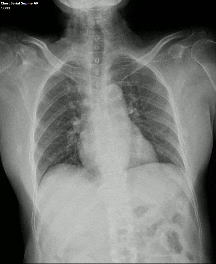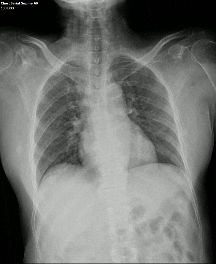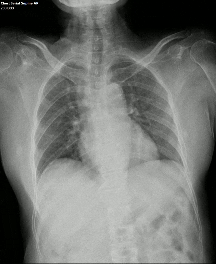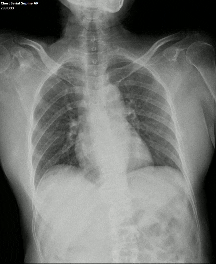
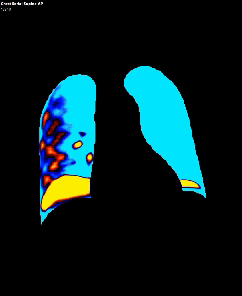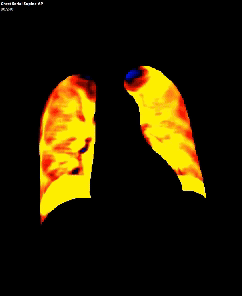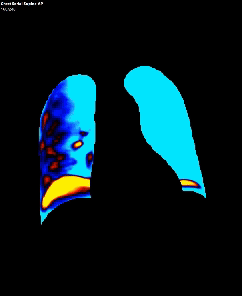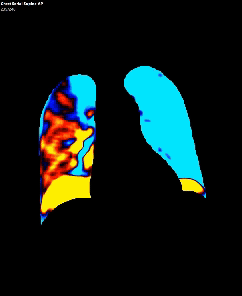
Another limiting factor was the presence of associated conditions like cardiomegaly, post operative changes (eg.: adhesions etc.), pleural effusion which caused under assessment of the corresponding lung field.