Neurosurgical Techniques: A Primer for Radiology Residents
Cranial neurosurgical approaches are commonly achieved through these main techniques: burr holes, craniotomy and craniectomy [1,2]. 
- Burr Holes: small holes used for drainage, biopsy, or intracranial monitoring. They appear as well-defined defects on CT, with associated fluid, hemostatic material, bone dust or even air immediately postoperatively.
- Craniotomy: creating a skull flap that's replaced and fixed with sutures or titanium plates at the end. Early imaging shows scalp swelling, extra-axial fluid collections, and pneumocephalus. Initially, the bone flap has sharp margins that remodel over time.
- Craniectomy: used for decompression in case of trauma or stroke, without replacing the bone flap. It results in the fusion of the dura and galea, appearing as a smooth hyperattenuating and mildly enhancing line (meningogaleal complex) [3].
Choosing the right imaging modality
CT is the primary modality for early evaluation of complications due to its availability, speed and ability to show life-threatening complications, such as haemorrhage, tension pneumocephalus, and cerebral oedema. Meanwhile, early MRI is more sensitive to ischemic and infectious complications and can delineate the extent of resection after brain tumour resection.
Resection cavity
Early postoperative MRI is essential for careful evaluation of the resection cavity and should be performed within 24 to 48h after surgery to keep reactive enhancement at a minimum [4,5]. The surgical bed is typically lined with hemostatic agents containing variable amounts of CSF and blood products. Around the resection cavity, some areas of restricted diffusion on early postoperative MRI represent devitalised brain tissue. There may also be some vasogenic oedema, which shows facilitated diffusion and is related to intraoperative retraction of brain parenchyma. The most crucial factor for assessing the extent of resection for high-grade gliomas or metastases is contrast enhancement in the cavity: some linear, thin enhancement may occur on the margins of the cavity, representing reactive change, and any thick, irregular and nodular enhancing areas should raise suspicion for tumour rest. However, this applies only to early MRI, performed within 72h from surgery, because as the fibroinflammatory process progresses, there will be diffuse and irregular enhancement, sometimes seen even for non-tumoral pathology. Over time, a normal resection cavity will shrink and collapse [1]. 

Complications
The most common post-resection complications are haemorrhage and iatrogenic stroke. Other complications, like infections, CSF fistulas and tension pneumocephalus, are rarer but require prompt diagnosis and management. We provide an easy framework for radiology residents to check for complications. 
A. Pneumocephalus represents intracranial air accumulation after surgery, with some amount of subdural and intraparenchymal air considered benign and can be managed conservatively [6]. When subdural air causes compression of the cerebral parenchyma, it defines tension pneumocephalus, a neurosurgical emergency associated with worsening neurological symptoms [7]. The primary modality for assessment is CT, which can depict variable amounts of free air in the epicranial, epidural, subdural, intraparenchymal and intraventricular spaces. This is considered a normal finding on early postoperative imaging; however, symptomatic or increasing pneumocephalus should raise concerns. Tension pneumocephalus should be suspected in case of neurological deterioration. CT scan demonstrates bilateral extra-axial frontal air accumulation with compression of the cerebral parenchyma (peaking) and separation of the frontal lobes from the falx (“Mount Fuji sign”) [3,8,9]. MRI has inferior sensitivity: air appears dark on most sequences and can be mistaken for flow-voids or blood products.
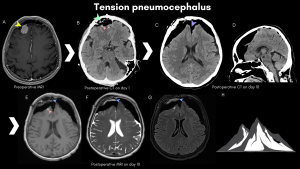
B. Blood products are another common early finding in postoperative neuroimaging involving the operative bed and structures along the surgical corridor. CT and MRI are useful in detecting blood products, with a higher sensibility and sensitivity for SWI-MRI. Small foci of blood and haemostatic material in and around the surgical cavity are expected, appearing as hyperdense spots on CT, respectively, T1 hyperintense and SWI hypointense foci on MRI. 
Large and expanding intracerebral hematomas can occur due to vascular injury during surgery, causing oedema and raised intracranial pressure, requiring immediate reintervention. Blood is more commonly found in the subdural and epidural spaces, sometimes with associated subarachnoid haemorrhage [10]. It is important to differentiate between subdural haemorrhages - crescentic, crossing suture lines, and epidural haemorrhages - lentiform and do not cross suture lines. SDH have a more favourable prognosis when <10mm with less than 5mm midline shift. Attention should be given not to overdiagnose remnant subdural fluid collections such as hematomas after surgery. 
Distant haemorrhage is a rare neurosurgical complication that occurs away from the surgical site, most frequently in the cerebellum early after supratentorial craniotomy, with a mortality rate of up to 25% [11]. The underlying pathophysiology for this unique pattern of haemorrhage is sudden CSF loss, leading to cerebellar sagging with subsequent compression of the superior cerebellar veins and, ultimately, hemorrhagic venous infarct [12, 13]. 
C. Ischemic stroke is a common early neurosurgical complication related to dysregulation of vascular circulation perioperatively, the underlying pathology and surgical techniques.
MRI-DWI is more sensitive in detecting postoperative strokes than CT, showing areas of hyperintensity on DWI with corresponding hypointensity on the ADC map. Thin rims of DWI hyperintensity at the resection borders don't constitute ischemic lesions but expected post-surgical findings [11,14]. 
Vascular lesions involve main branches of intracranial arteries, small terminal branches around the resection bed or venous territories.
Tumours near the dural venous sinuses increase the risk of sinus breach with venous sinus thrombosis. Venous infarctions are characterised by flame-shaped hemorrhagic transformation at the grey-white matter interface, with venous filling defects highlighted after contrast administration [11]. 
D. Any displacement of cerebral structures should be carefully evaluated postoperatively in comparison to preoperative imaging. The most serious complication is brain herniation (subfalcine, transtentorial, central, tonsillar and external) due to oedema, haemorrhage, and increased intracranial pressure.
There are also complications specific to each neurosurgical technique:
- Pseudomeningocele: an extradural CSF collection due to herniation of the subarachnoid space in the absence of dural closure, usually after posterior fossa craniectomies [15].
- Sinking Skin Flap Syndrome (Paradoxical herniation syndrome): a late post-craniectomy complication occurring when intracranial pressure drops below atmospheric pressure, leading to an inward shift of the brain and cutaneous tissues towards the contralateral side [16].
- Burr Hole Plunging: a rare intraoperative complication where the drill penetrates deeper than intended, potentially causing dural tears, brain lacerations, or vascular injury [17].


E. Vasogenic oedema is common post-surgery, appearing as white matter hypodensities (CT) or T2/FLAIR hyperintensities without restricted diffusion. Cytotoxic oedema appears as restricted diffusion with loss of grey-white matter differentiation and sulcal effacement. Mild oedema typically resolves gradually, while extensive oedema can raise intracranial pressure with consequent brain herniation and hypoxia. 
F. During neurosurgery, vascular injuries, dural defects and CSF leakage can give rise to multiple types of fistulas. CSF fistulas are the most frequent type, arising after skull base surgeries involving the sphenoid bone and mastoid cells, resulting in CSF otorrhea and/or rhinorrhea. MRI is the modality of choice when a CSF fistula is suspected, with high-resolution 3D T2 sequences offering a clear view of CSF leaking from the surgical site through bony defect into the nasal cavity or mastoid cells, internal or middle ear [19].
G. Postoperative infections are most commonly caused by inoculation of skin flora and can involve any structure from the wound towards the intracranial space. They usually have a subacute clinical course, requiring imaging for prompt diagnosis and characterisation:
- Cellulitis: infection of the skin and superficial fascia; imaging demonstrates deep extension.
- Bone flap osteomyelitis: CT reveals bone destruction with multiple lytic areas and overlying scalp inflammatory changes; MRI shows marrow edema and decreased T1 signal.
- Meningitis: MRI may show meningeal enhancement and restricted diffusion.
- Extradural abscess: lentiform collection with thickened enhancing dura under the craniotomy flap; may show restricted diffusion.
- Subdural Empyema: crescentic collection with peripheral enhancement and restricted diffusion, often with mass effect.
- Cerebral Abscess: ring-enhancing lesion with diffusion restriction.
- Pyogenic Ventriculitis: ventricular debris, periventricular enhancement, and restricted diffusion [2].

