Results
The study included 20 patients with acute GV, all of whom had a previous radiological diagnosis of HH (mean age: 71.90 years; 52% women). The mean time since HH diagnosis was 4.71 years and the most frequent type of HH prior GV was sliding.
The flowchart of the study is shown in figure 3.
Fig 3: Flowchart followed in the study.
Table 1 shows the sociodemographic characteristics, as well as the type of HH and GV in the sample.
Table 1: Sociodemographic characteristics, hiatal hernia characteristics, and type of GV in the sample. HH: hiatal hernia. GV: gastric volvulus. R1: evaluating radiologist 1. R2: evaluating radiologist 2. S: sliding. U-D: upside-down. M: Morgagni hernia. EGDT: esophagogastroduodenal transit. CT: computed tomography. PET: positron emission tomography. Rx: plain radiograph. O-A: organoaxial. M-A: mesenteroaxial. ^Time since HH diagnosis (in years).
To date, the largest sample of GV cases published in the literature does not exceed 40 cases [9,10]. This study presents the third-largest sample of cases reported, with the special consideration that all cases were confirmed intraoperatively or through independent analysis by two radiologists and had a prior imaging test demonstrating HH or diaphragmatic hernia, as one of the most frequently involved risk factors in the development of GV is the previous presence of HH [11]. Regarding the classification of GV types, the most commonly used is the topographic classification, which is based on the main axis of rotation and describes two types: organoaxial (in which rotation occurs around a longitudinal axis, with the greatest curvature above the lesser curvature), and mesenteroaxial (where rotation occurs around the axial axis of the stomach, with the gastroesophageal junction located below the gastroduodenal junction) [6, 12-15]. In our series, according to an expert in abdominal radiology, all cases of volvulus were mesenteroaxial type. Despite its validity and frequent use, the topographic classification does not explain the pathophysiology of GV and has the disadvantage of being difficult to interpret in radiological images. In our case, the pathophysiological mechanism of ‘back-and-forth stomach’ requires specific consideration: gastric contents (partial or total) would herniate through the diaphragmatic defect instead of the esophageal hiatus, leaving the gastroesophageal junction in its usual location.
Table 2 describes the clinical and laboratory parameters at acute presentation, including complications, image findings, treatment and patients‘ outcomes. The most frequent symptom was epigastric pain, present in all cases, followed by vomiting (90%). The most common blood test abnormalities were leukocytosis and elevated C-reactive protein. Complications occurred in up to half of the cases, with a 20% mortality rate, which aligns with figures reported in the literature, underscoring the importance of CT in guiding urgent treatment decisions [5,15].
Table 2: Clinical and laboratory data, radiological complications, and patients’ evolution in the sample. *1: epigastric pain; 2: retching without vomiting; 3: intolerance to nasogastric tube; 4: vomiting; 5: dehydration (tachycardia and/or hypotension, dry mucous membranes). **Patient discharged after GV diagnosis, readmitted 3 months later for a new episode treated conservatively, in which he died.
Bivariate analysis comparing GV with and without signs of radiological complications showed that a duration of symptoms exceeding 24 hours before the CT scan in the acute episode, was significantly associated with the presence of radiological complications (p = 0.011). Table 3.
Table 3: Bivariate analysis for the dependent variable ‘presence of radiological complications’. HH: hiatal hernia. NGT: nasogastric tube. CRP: C-reactive protein. LDH: lactate dehydrogenase. *Significant value.
Other contributing factors, such as the degree of gastric dilation or patient age, were not found to be significant in our case. This could be attributed, among other reasons, to the small sample size. Therefore, studies with larger cohorts are needed to evaluate these and other potential factors, providing a more comprehensive understanding of the elements that influence radiological complications. This would help develop more effective intervention strategies to prevent these complications in patients. Figure 4.
Fig 4: Illustrative examples of complicated GV in the study. A) Sagittal image (soft tissue window) of a GV with microperforation of the gastric fundus (extraluminal bubble indicated by the thin arrow) and perigastric fluid (white arrow). B) Axial image (lung window) showing scattered extraluminal gas bubbles (black arrow). C, D) Coronal and axial images (soft tissue window) of a GV with abundant peritoneal free fluid (asterisks) predominantly subhepatic. E) Gastric pneumatosis (white arrows) and parietal hypodensity of the reherniated fundus in the abdomen (yellow arrow). F) Portal pneumatosis (black arrows). G, H) Axial images (lung window and soft tissue in G and H, respectively) showing abundant pneumoperitoneum and a large continuity defect in the left wall of the gastric fundus, related to perforation.
Characteristic manifestations of 'back-and-forth stomach' were observed in 95% of cases. Illustrative examples of GV from the patients included in the study are shown in figure 5.
Fig 5: Illustrative examples of GV in the study. A, B, C) Organoaxial GV. A) Axial non-contrast CT image showing the antrum (a) and fundus (f) in the thoracic cavity. B) Coronal non-contrast CT image showing the reherniated fundus in the abdominal cavity through the esophageal hiatus, identifying the hernial neck (white arrow). C) Coronal contrast-enhanced CT image of a GV visualizing the hernia neck and gastroduodenal junction (dashed arrow). D, E, F) Mesenteroaxial GV. D) Coronal non-contrast CT image showing the stomach, with herniated antrum (a) in the thoracic cavity and distended body and fundus (f) in the abdominal cavity. E) Sagittal non-contrast CT image showing the herniated antrum and anthropyloric junction (asterisk) in the thorax, entering below the diaphragm. F) Sagittal non-contrast CT image showing the body and fundus distended in the abdominal region in the context of GV.
Figure 6 presents cases of GV that were incorrectly diagnosed as false positives in the radiology report.
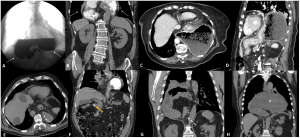
Fig 6: Examples that were incorrectly diagnosed as GV (false positives) in the radiological report. A, B) Patient with epigastric pain, without significant analytical alterations, diagnosed with acute GV on CT. It corresponded to a type IV hernia, previously diagnosed as shown in fluoroscopy in figure A (arrows). The patient refused surgery and was treated conservatively, with no complications. C, D) Patient with vomiting and abdominal discomfort who was wrongly diagnosed with GV. The images show a large sliding hiatal hernia, moderately dilated and containing food. The patient refused surgery and follow-up images one month later (D), without symptoms, showed the same findings. E, F) Patient with epigastric pain and laboratory abnormalities erroneously diagnosed as ‘probably volvulated hiatal hernia’. The images show a large, non-dilated sliding hiatal hernia that allows the passage of the nasogastric tube (arrow in E) and the passage of contrast to the duodenum (yellow arrow in F). G) Patient with epigastric pain and vomiting diagnosed with GV. The image shows a right diaphragmatic hernia (orifice indicated by the dashed arrow), not dilated and without inflammatory signs (solid arrows). Conservative treatment was decided given the limited symptoms and rapid clinical improvement. H) Patient with abdominal pain and vomiting wrongly diagnosed with GV. The coronal image shows a dilated type IV hernia with fluid content inside (asterisk). The patient improved after nasogastric catheterization, and a control CT scan 3 months later showed persistence of the hernia, without signs of complications.
Additionally, figure 7 includes illustrative examples of the radiological signs proposed for GV in our sample. These signs are based on a retrospective review by the author; however, none are pathognomonic. Their presence should be futher evaluated in future studies.
Fig 7: Examples of the radiological signs evaluated in the sample. A, B) The ‘Long-beak duck’ sign. C, D) The ‘Windswept trousers’ sign. E) The ‘Three circles within the hiatus’ sign. F, G) The ‘Gastroesophageal uterus’ sign.
Based on the data obtained in our case series and previous studies, we reinforce the ‘back-and-forth stomach’ theory [1], which consistently, clearly, and simply explains the pathophysiological mechanism of GV. This understanding enables radiologists to confidently recognize and diagnose this condition.
The main strength of this study lies in its multicenter design, which is particularly important given the low incidence of acute GV. However, the study has certain limitations. Its retrospective nature and the absence of a control group may affect the validity of some uncollected variables (primarily clinical but also analytical). Additionally, the relatively small sample size remains a limitation. Further studies with larger cohorts are needed to validate the proposed pathophysiological hypothesis.







